Sensitization of Non-M3 Acute Myeloid Leukemia Blasts to All-Trans Retinoic Acid by the LSD1 Inhibitor Tranylcypromine: TRANSATRA Phase I Study
- PMID: 40459012
- PMCID: PMC12319880
- DOI: 10.1111/ejh.14426
Sensitization of Non-M3 Acute Myeloid Leukemia Blasts to All-Trans Retinoic Acid by the LSD1 Inhibitor Tranylcypromine: TRANSATRA Phase I Study
Abstract
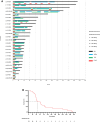
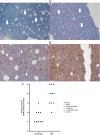
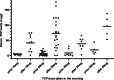
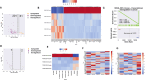
The treatment of elderly, nonfit acute myeloid leukemia (AML)/MDS patients with relapsed/refractory (R/R) disease remains challenging. As histone demethylase LSD1 (KDM1A) is a rational therapeutic target in AML, we conducted a phase I trial ("rolling-six design") with the LSD1 inhibitor tranylcypromine (TCP, dose levels [DL] 20, 40, 60, 80 mg p.o. d1-28) combined with fixed-dose ATRA (45 mg/m2 p.o. d10-28) and low-dose cytarabine (LDAC, 40 mg s.c. d1-10). The primary endpoint was dose-limiting toxicity (DLT) in the first 28 days of treatment. The aim was the determination of the maximum tolerated TCP dose (MTD). Twenty-three patients with AML and 2 with MDS were accrued. TCP was administered for a median of 39.5 days (range: 11-228). No DLTs were observed at any DL; MTD could not be established. No differentiation syndrome occurred. Two patients attained a PR; SD was achieved in 10 of 22 evaluable patients. Median OS was 62 days (range: 14-325). Accompanying studies included pharmacokinetics, serial determinations of fetal hemoglobin (HbF), detection of CD38 upregulation with treatment, as well as transcriptome changes in purified blood blasts over time. In conclusion, the combination of TCP with ATRA and LDAC was well feasible, even at the highest DL. Hence, studies with more potent LSD1 inhibitors appear warranted. Trial Registration: German Clinical Trials Register (DRKS): DRKS00006055. For further Information see https://drks.de/search/en/trial/DRKS00006055.
Keywords: CD38; LSD1; chromatin; differentiation; histone demethylase; myelodysplastic syndrome.
© 2025 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Tchekmedyian R., Elson P., Gerds A. T., et al., “Analysis of Outcomes of Patients With Relapsed/Refractory Acute Myeloid Leukemia Treated in Randomized Clinical Trials,” Blood 128, no. 22 (2016): 4000, 10.1182/blood.V128.22.4000.4000. - DOI
-
- Stomper J., Rotondo J. C., Greve G., and Lübbert M., “Hypomethylating Agents (HMA) for the Treatment of Acute Myeloid Leukemia and Myelodysplastic Syndromes: Mechanisms of Resistance and Novel HMA‐Based Therapies,” Leukemia 35, no. 7 (2021): 1873–1889, 10.1038/s41375-021-01218-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

