Apolipoprotein-L1 G1 variant contributes to hydrocephalus but not to atherosclerosis in apolipoprotein-E knock-out mice
- PMID: 40459058
- PMCID: PMC12236173
- DOI: 10.1042/CS20255324
Apolipoprotein-L1 G1 variant contributes to hydrocephalus but not to atherosclerosis in apolipoprotein-E knock-out mice
Abstract
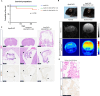
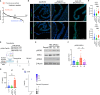
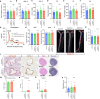
In USA, six million individuals with Sub-Saharan ancestry carry two APOL1 high-risk variants, which increase the risk for kidney diseases. Whether APOL1 high-risk variants increase other diseases under dyslipidemia remains unclear and requires further investigation.We characterized a mouse model to investigate the role of APOL1 in dyslipidemia and cardiovascular diseases. Transgenic mice carrying APOL1 (G0 and G1 variants)on bacterial artificial chromosomes (BAC/APOL1 mice) were crossed with the ApoE knock-out (ApoE-KO) dyslipidemia and atherosclerosis mouse model. The compound transgenic mice were evaluated for the impact of APOL1 on systemic phenotypes. ApoE-KO mice carrying APOL1-G0 and APOL1-G1 did not show differences in the extent of atherosclerotic lesions or aortic calcification, as evaluated by Sudan IV staining and radiographic examination, respectively. However, ~20% of ApoE-KO; BAC/APOL1-G1 mice developed hydrocephalus and required euthanasia. The hydrocephalus was communicating and likely was due to excess cerebrospinal fluid produced by the choroid plexus, where epithelial cells expressed APOL1. Single-nuclear RNA-seq of choroid plexus identified solute transporter upregulation and mTORC2 pathway activation in APOL1-G1-expressing epithelial cells. Further, in the All of Us cohort, we found higher hydrocephalus prevalence among individuals with the APOL1-G1 variant in both recessive and dominant models, supporting the mouse findings. While APOL1-G1 expression in ApoE-KO mice did not worsen cardiovascular disease phenotypes, we uncovered hydrocephalus as a novel APOL1 risk allele-mediated phenotype. These findings extend the spectrum of APOL1-associated pathologies.
Keywords: APOL1; apolipoproteins; atherosclerosis; hydrocephalus; transcriptomics.
© 2025 The Author(s).
Figures


Update of
-
Apolipoprotein-L1 G1 variant contributes to hydrocephalus but not to atherosclerosis in apolipoprotein-E knock-out mice.bioRxiv [Preprint]. 2024 Dec 28:2024.12.28.630625. doi: 10.1101/2024.12.28.630625. bioRxiv. 2024. Update in: Clin Sci (Lond). 2025 May 27:CS20255324. doi: 10.1042/CS20255324. PMID: 39803526 Free PMC article. Updated. Preprint.
References
Grants and funding
- OT2 OD026556/OD/NIH HHS/United States
- U2C OD023196/OD/NIH HHS/United States
- OT2 OD025315/OD/NIH HHS/United States
- OT2 OD026551/OD/NIH HHS/United States
- U24 OD023121/OD/NIH HHS/United States
- OT2 OD026552/OD/NIH HHS/United States
- OT2 OD026549/OD/NIH HHS/United States
- OT2 OD025337/OD/NIH HHS/United States
- OT2 OD025277/OD/NIH HHS/United States
- OT2 OD026555/OD/NIH HHS/United States
- OT2 OD026550/OD/NIH HHS/United States
- OT2 OD026553/OD/NIH HHS/United States
- OT2 OD023205/OD/NIH HHS/United States
- OT2 OD025276/OD/NIH HHS/United States
- OT2 OD026557/OD/NIH HHS/United States
- OT2 OD026554/OD/NIH HHS/United States
- U24 OD023163/OD/NIH HHS/United States
- OT2 OD023206/OD/NIH HHS/United States
- U24 OD023176/OD/NIH HHS/United States
- OT2 OD026548/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

