High-Throughput Drug Screening of Clear Cell Ovarian Cancer Organoids Reveals Vulnerability to Proteasome Inhibitors and Dinaciclib and Identifies AGR2 as a Therapeutic Target
- PMID: 40459063
- PMCID: PMC12188421
- DOI: 10.1158/2767-9764.CRC-25-0024
High-Throughput Drug Screening of Clear Cell Ovarian Cancer Organoids Reveals Vulnerability to Proteasome Inhibitors and Dinaciclib and Identifies AGR2 as a Therapeutic Target
Abstract
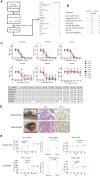
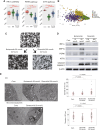
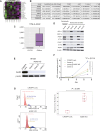
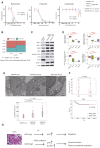
There are currently no effective treatments available for clear cell ovarian cancer (CCC). In this study, we aimed to identify effective drugs for CCC through high-throughput drug screening (HTDS) using ovarian cancer organoids and determine novel therapeutic targets based on the biological characteristics of CCC through omics analysis. An ovarian cancer organoid biobank was established, and HTDS was conducted using CCC organoids based on libraries of 361 and 4,560 compounds. The efficacy of the identified drugs was verified in in vitro and in vivo experiments using a patient-derived organoid xenograft mouse model. Transcriptome analysis was performed to identify genes related to the pathways targeted by the identified drugs in CCC and to assess their potential as therapeutic targets. Proteasome inhibitors and dinaciclib were extracted using HTDS and shown to inhibit tumorigenesis in vitro and in vivo. CCC, like multiple myeloma, exhibited activated endoplasmic reticulum (ER) stress and unfolded protein response (UPR), and treatment with proteasome inhibitors further enhanced ER stress and UPR, ultimately leading to cell death. Transcriptome analysis identified anterior gradient-2 (AGR2) as a key gene involved in UPR in CCC. CRISPR knockout of AGR2 suppressed cell proliferation, increased sensitivity to proteasome inhibitors, and reversed platinum resistance in CCC. AGR2 knockout also upregulated Schlafen 11, contributing to platinum sensitivity. ER stress and the UPR are activated in CCC, and proteasome inhibitors disrupt this balance, ultimately leading to cell death. AGR2 may serve as a potential therapeutic target in CCC.
Significance: Proteasome inhibitors and dinaciclib are identified as effective drugs for CCC. CCC has a high basal UPR, and proteasome inhibition may disrupt this balance. AGR2 is involved in the UPR of CCC, and inhibiting AGR2 further enhances the UPR and confers platinum sensitivity, making it a potential therapeutic target.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
T. Yoshimura reports grants from JSR Corporation and The Keio University Doctorate Student Grant-in-Aid Program during the conduct of the study, as well as a patent to PCT/JP2023/028482 pending. M. Takahashi reports grants and nonfinancial support from JSR Corporation during the conduct of the study. D. Aoki reports personal fees from AstraZeneca, MSD, Chugai, Eisai, Takeda, and Myriad Genetics outside the submitted work. T. Chiyoda reports grants from JSR Corporation during the conduct of the study; grants from Takeda outside the submitted work; and a patent to PCT/JP2023/028482 pending. No disclosures were reported by the other authors.
Figures


References
-
- Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Mikami M, et al. A retrospective study for investigating the relationship between old and new staging systems with prognosis in ovarian cancer using gynecologic cancer registry of Japan Society of Obstetrics and Gynecology (JSOG): disparity between serous carcinoma and clear cell carcinoma. J Gynecol Oncol 2020;31:e45. - PMC - PubMed
-
- Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000;88:2584–9. - PubMed
-
- Kuroda Y, Chiyoda T, Kawaida M, Nakamura K, Aimono E, Yoshimura T, et al. ARID1A mutation/ARID1A loss is associated with a high immunogenic profile in clear cell ovarian cancer. Gynecol Oncol 2021;162:679–85. - PubMed
-
- Heinze K, Nazeran TM, Lee S, Krämer P, Cairns ES, Chiu DS, et al. Validated biomarker assays confirm that ARID1A loss is confounded with MMR deficiency, CD8+ TIL infiltration, and provides no independent prognostic value in endometriosis-associated ovarian carcinomas. J Pathol 2022;256:388–401. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

