COP I vesicles facilitate classical swine fever virus proliferation by transporting fatty acid synthase from the Golgi apparatus to the endoplasmic reticulum
- PMID: 40459259
- PMCID: PMC12282108
- DOI: 10.1128/jvi.00305-25
COP I vesicles facilitate classical swine fever virus proliferation by transporting fatty acid synthase from the Golgi apparatus to the endoplasmic reticulum
Abstract
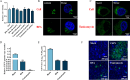
Classical swine fever virus (CSFV) is an enveloped, positive-sense, single-stranded RNA virus in the Flaviviridae family that remodels the cell's endomembrane for its own propagation. The early secretory pathway is exploited by viruses for their lifecycle, but the mechanism underlying this hijacking of the early secretory pathway in CSFV infection remains unknown. Here, we observed that disrupting the functions of the early secretory pathway organelles, the Golgi apparatus, the endoplasmic reticulum (ER), and coatomer protein I (COP I) vesicles and coatomer protein II (COP II) vesicles resulted in a significant inhibition of CSFV propagation. Further, we revealed that COP I vesicles were required for CSFV RNA replication, but not for the formation of viral replication complexes. The results support the hypothesis that participation of COP I vesicles in viral RNA replication involves their capacity for cargo trafficking. Intact COP I vesicles were isolated and subjected to data-independent acquisition quantitative proteomics analysis to identify the differences in the proteomes of COP I vesicles. This analysis revealed an increase in fatty acid synthase (FASN), a critical factor for CSFV RNA replication, within COP I vesicles, while its presence in COP II vesicles decreased in CSFV-infected cells. Meanwhile, blocking COP I vesicle formation resulted in decreased levels of FASN in the ER, impairing CSFV RNA replication. Collectively, we provide evidence that COP I vesicles mediate FASN trafficking from the Golgi apparatus to the ER to facilitate CSFV RNA replication, which advances our understanding of the role of the early secretory pathway in CSFV proliferation.IMPORTANCEClassical swine fever is a highly contagious disease caused by the classical swine fever virus (CSFV) that infects domestic pigs and wild boars and results in significant economic losses to the swine industry. The early secretory pathway in host cells has often been hijacked by viruses for viral genome replication, assembly, and release of virions. Here, our data revealed that the function of early secretory pathway organelles such as the endoplasmic reticulum (ER) and the Golgi apparatus, and the membrane-bound transport intermediates, COP I vesicles and COP II vesicles, that facilitate transport, were involved in CSFV proliferation in PK-15 cells. Our findings demonstrate that COP I vesicles significantly promote CSFV RNA replication by trafficking fatty acid synthase from the Golgi apparatus to the ER. Our data suggest that manipulation of early secretory pathway function in target host cells could provide a promising strategy for a novel anti-CSFV therapeutic.
Keywords: classical swine fever virus; coatomer protein I; coatomer protein II; early secretory pathway; fatty acid synthase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

