Persistence with anti-dementia medications: a systematic review and meta-analysis
- PMID: 40459346
- PMCID: PMC12131239
- DOI: 10.1093/ageing/afaf151
Persistence with anti-dementia medications: a systematic review and meta-analysis
Abstract
Background: Suboptimal persistence with anti-dementia drugs (ADDs) in patients with dementia is associated with poorer clinical outcomes, including accelerated disease progression, cognitive decline and increased healthcare utilisation. This study aimed to systematically review real-world persistence rates with ADDs and identify factors influencing persistence.
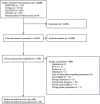
Methods: We followed the Cochrane methodology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and searched Medline, Embase, PsycINFO and CINAHL from 1 January 1995 to 5 February 2024. Pooled persistence rates were calculated using random-effects Mantel-Haenszel models. Heterogeneity was assessed using I2 statistics, publication bias via funnel plots and Egger's/Begg's tests, and moderators were explored through meta-regression.
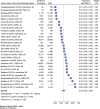
Results: We included 68 studies involving 684,493 participants aged 50 years and older who received ADD. The mean 12-month persistence rate was 49% (95% CI: 42%-56%). Subgroup analyses revealed higher persistence for studies where a permissible gap for medicine refills was not required based on the methodology (67%, 95% CI: 38%-90%), those examining memantine (61%, 95% CI: 38%-82%), studies published between 2011 and 2015 (54%, 95% CI: 41%-68%) and studies conducted in Europe (57%, 95% CI: 43%-71%). Of these, the permissible gap remained an independent predictor of between-study heterogeneity in persistence (β = 0.36, 95% CI: 0.18-0.54).
Conclusion: The meta-analysis demonstrated relatively low persistence to ADDs, which varied according to the evaluation criteria used. Targeted interventions to improve persistence with therapy may lead to better outcomes in patients with dementia. Also, a standardised framework for measuring persistence could improve research reliability.
Keywords: anti-dementia drugs; dementia; medication persistence; older people; systematic review.
© The Author(s) 2025. Published by Oxford University Press on behalf of the British Geriatrics Society.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Withdrawal or continuation of cholinesterase inhibitors or memantine or both, in people with dementia.Cochrane Database Syst Rev. 2021 Feb 3;2(2):CD009081. doi: 10.1002/14651858.CD009081.pub2. Cochrane Database Syst Rev. 2021. PMID: 35608903 Free PMC article.
-
Donepezil for dementia due to Alzheimer's disease.Cochrane Database Syst Rev. 2018 Jun 18;6(6):CD001190. doi: 10.1002/14651858.CD001190.pub3. Cochrane Database Syst Rev. 2018. PMID: 29923184 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis.Cochrane Database Syst Rev. 2021 Feb 22;2(2):CD013306. doi: 10.1002/14651858.CD013306.pub2. Cochrane Database Syst Rev. 2021. PMID: 33704781 Free PMC article.
-
Antihypertensive withdrawal for the prevention of cognitive decline.Cochrane Database Syst Rev. 2016 Nov 1;11(11):CD011971. doi: 10.1002/14651858.CD011971.pub2. Cochrane Database Syst Rev. 2016. PMID: 27802359 Free PMC article.
References
-
- Dementia Key Facts. World Health Organization. 2024. https://www.who.int/news-room/fact-sheets/detail/dementia (31 January 2024, date last accessed).
-
- Alzheimer’s Disease International World Alzheimer Report 2010, the Global Economic Impact of Dementia. Alzheimer’s Disease International (ADI); 2010:56. https://www.alzint.org/u/WorldAlzheimerReport2010.pdf. (31 January 2024, date last accessed).
-
- Alzheimer’s Disease International , World Alzheimer Report 2015 the Global Impact of Dementia an Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International (ADI); 2015:87. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf. (31 January 2024, date last accessed).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous