Insurer and patient costs for repeat breast surgery after initial lumpectomy for breast cancer
- PMID: 40459675
- PMCID: PMC12209022
- DOI: 10.1007/s10549-025-07735-1
Insurer and patient costs for repeat breast surgery after initial lumpectomy for breast cancer
Abstract
Purpose: ~ 14-25% of patients who undergo a primary lumpectomy for the treatment of breast cancer require a reoperation due to adverse outcomes like positive surgical margins or early cancer recurrence, adding burden to the patients, providers, and payors. We analyze the economic impact of patients who require repeat breast tissue resection as part of their treatment following initial resection.
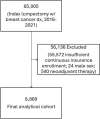
Methods: We utilized the Merative™ MarketScan Research Database to identify a cohort of women in the United States who received an index lumpectomy between 2016 and 2021 and identified their healthcare encounters one year postoperatively, including any repeat lumpectomies or mastectomies, as well as the use of any intraoperative adjuncts (e.g. localization methods or frozen sections).
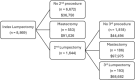
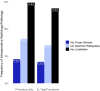
Results: Among 8,869 patients with a primary lumpectomy, 25% (n = 2197) underwent a second surgery, of which 75% (n = 1644) was a repeat lumpectomy and 25% (n = 553) was a mastectomy. Median healthcare expenditure for primary lumpectomy plus one year follow up was $55,985 USD ($2,500 out-of-pocket). Among patients with secondary procedures, median healthcare expenditure from primary lumpectomy plus one year follow up was $63,416 ($3,005 out-of-pocket) for repeat lumpectomy and $87,961 ($3,100 out-of-pocket) for subsequent mastectomy patients. Repeat procedures were more common among patients who did not receive an intraoperative adjunct for lesion localization or margin assessment.
Conclusion: While lumpectomy is the most common surgery for early-stage breast cancer, it often is not definitive, which can result in large added financial and operational burdens. Patient risk stratification and intraoperative adjuncts are needed to minimize risk of reoperation.
Keywords: Breast cancer; Economic evaluation; Lumpectomy; Surgery.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: SW, YL, and FZ are employees of Intuitive Surgical. For the remaining authors, no relevant conflicts are declared. Ethical approval: As this was an observational study of de-identified patients in the MarketScan database, Institutional Review Board approval and consent were exempt (in accordance with the Health Insurance Portability and Accountability Act Privacy Rule). An IRB exemption was approved by the wcg™ IRB (#13931684).
Figures
References
-
- Globocan 2022. In.: International Agency for Research on Cancer; 2024.
-
- Cancer Trends Progress Report: Stage at Diagnosis. In.: National Cancer Institute; 2024.
-
- Breast Cancer Facts & Figures (2019) 2019–2020. American Cancer Society, In.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical