Real‑World Experience of 1 Year of Tralokinumab Treatment in Adult Patients with Atopic Dermatitis: A Single-Center Retrospective Study
- PMID: 40459712
- PMCID: PMC12256368
- DOI: 10.1007/s13555-025-01445-8
Real‑World Experience of 1 Year of Tralokinumab Treatment in Adult Patients with Atopic Dermatitis: A Single-Center Retrospective Study
Abstract
Introduction: Atopic dermatitis (AD) is a chronic inflammatory disease requiring long-term management. Biologic therapies have emerged as systemic treatment options for AD, and real-world evidence (RWE) of their use is needed to inform optimal treatment decisions.
Methods: A retrospective, single-center case series was conducted including 37 adults with AD who were systemic-naive or inadequately responded to previous AD treatment and had a visit around 1 year after tralokinumab initiation prior to May 2024. Patient demographics, disease characteristics, and treatment history were collected. Outcomes of tralokinumab treatment, including investigator's global assessment (IGA) score, body surface area (BSA), and adverse events (AEs), were extracted from a 2-month visit (defined as 1-3 months) and a 1-year visit (defined as ≥ 10 months) after tralokinumab initiation.
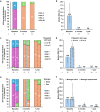
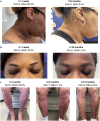
Results: Thirty-seven patients (median age, 58.0 years; 49% male) on tralokinumab for approximately 1 year or longer were included. At baseline, most patients (97% [33/34]) had moderate-to-severe AD (IGA 3 or 4), and median (interquartile range [IQR]) BSA was 10.0% (5.0%; 18.8%); 19% (7/37) of patients were biologic-experienced, having been previously on dupilumab. Most patients (95% [35/37]) transitioned to tralokinumab due to inadequate response to previous treatment. The proportion of patients with IGA score 0 or 1 increased from 0% (0/34) at baseline to 85% (22/26) after 2 months and 93% (28/30) at 1 year of tralokinumab treatment. Median (IQR) BSA improved to 0.5% (0.0%; 1.0%) and 0.0% (0.0%; 1.0%) at the 2-month and 1-year visits, respectively. Similar improvements were observed regardless of Fitzpatrick skin type or previous treatment history. No AEs were reported through 1 year of follow-up.
Conclusions: This large case series provides RWE building on tralokinumab clinical trial data and highlights the potential rapid and long-term response in patients with AD irrespective of their Fitzpatrick skin type or treatment history, including patients previously treated with dupilumab.
Keywords: Atopic dermatitis; Biologic therapies; Dupilumab; Real-world evidence; Tralokinumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Adrian O. Rodriguez is an investigator and consultant/advisor or speaker for: Abbvie, Arcutis, Bristol Myers Squibb, Eli Lilly, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, Sun, and UCB. Caid Sterling Smith has no conflict of interest. Ethical Approval: This case series was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The data were collected from the authors’ patient database within the authors’ private practice, Nashville Skin: Comprehensive Dermatology Center. Patients provided written informed consent to publish clinical details and photographs.
Figures
References
-
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22. - PubMed
-
- Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90(2):e43–56. - PubMed
-
- D. K., Schneider L, Asiniwasis RN, Boguniewicz M, De Benedetto A, Ellison K, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy. Ann Allergy Asthma Immunol. 2024;132(3):274–312. - PubMed
-
- ADBRY® (tralokinumab-ldrm) injection [prescribing information]. 2023; Madison, NJ: LEO Pharma Inc. [updated 12/2023].
LinkOut - more resources
Full Text Sources
Miscellaneous

