Monovalent mRNA XBB.1.5 vaccine effectiveness against COVID-19 hospitalization in Quebec, Canada: Impact of variant replacement and waning protection during 10-month follow-up
- PMID: 40460407
- PMCID: PMC12133164
- DOI: 10.1371/journal.pone.0325269
Monovalent mRNA XBB.1.5 vaccine effectiveness against COVID-19 hospitalization in Quebec, Canada: Impact of variant replacement and waning protection during 10-month follow-up
Abstract
Background: Vaccine formulations targeting contemporaneous subvariants have been developed to respond to SARS-CoV-2 virus evolution. Updated monovalent COVID-19 vaccines targeting the Omicron XBB.1.5 variant (XBB-vaccines) were administered in the province of Quebec, Canada, during 2023 autumn and 2024 spring vaccination campaigns. Our objective was to evaluate mRNA XBB-vaccine effectiveness (VE) against COVID-19 hospitalizations among adults aged ≥60 years overall during a ten-month follow-up period, by subvariant predominant period, and by time since vaccination.
Methods: We conducted a test-negative case-control study using Quebec population-based administrative data. Specimens collected from individuals aged ≥60 years tested at an acute-care hospital from October 2023 to August 2024 were considered test-positive cases if hospitalized for COVID-19, or controls if test-negative for SARS-CoV-2. Vaccination was defined by receipt of at least one mRNA XBB-vaccine (autumn or spring) dose. Subvariant predominant periods were defined according to whole-genome sequencing data from provincial laboratories: XBB or EG.5 and subvariants (XBB period), BA.2.86, JN.1 or subvariants (JN period), and KP.2 or KP.3 and subvariants (KP period). Multivariable logistic regression analyses estimated VE relative to several comparator groups, primarily those last-vaccinated in 2022, by subvariant period, by time since XBB-vaccination and by number of XBB-vaccine doses (KP period).
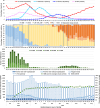
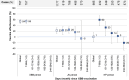
Results: Participants overall and by XBB, JN and KP periods included: 5532 (4.9%) test-positive cases (1321, 1838 and 1372, respectively) and 108473 (95.1%) test-negative controls (12881, 53414 and 28595, respectively); 14584 specimens were collected during periods of subvariant cocirculation. By subvariant period, 3322 (25.8%), 27041 (50.6%) and 15401 (53.9%) controls, respectively, were considered XBB-vaccinated. Overall VE was 30% (95%CI:24-35) and by XBB, JN or KP period: 54% (95%CI:46-62), 23% (95%CI:13-32) and 0% (95%CI:-18-15), respectively. During each subvariant period, the hospitalization risk was reduced only during the first four months post-vaccination.
Conclusions: Among individuals aged 60 years or older, mRNA XBB-vaccination provided meaningful, albeit limited to first four months post-vaccination, protection against COVID-19 hospitalization due to XBB, JN and KP subvariants. Better vaccines are needed to effectively protect older adults against COVID-19 hospitalizations.
Copyright: © 2025 Carazo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: SC, IGI and JP report funding from the Ministère de la santé et des services sociaux du Québec to conduct this work, paid to their institution. DMS reports funding from Public Health Agency of Canada and British Columbia Center for Diseases Control Foundation for Public Health for influenza and COVID-19 studies but not pertaining to the current study; from Michael Smith Foundation for Health Research and from Canadian Institutes of Health Research for COVID-19 studies but not pertaining to the current study; all grants were paid to her institution. Underlying data cannot be shared publicly by authors, because it belongs to the Ministère de la santé et des services sociaux du Québec and data access to researchers was given under the legal mandate of the Quebec National Director of Public Health. A request for data should be addressed to the research access centre designated by Quebec Government in accordance with the act respecting health and social services information (https://statistique.quebec.ca/en/institut/services-for-researchers/data).
Figures

References
-
- Health Canada. Health Canada authorizes Pfizer-BioNTech COVID-19 vaccine targeting the Omicron XBB.1.5 subvariant. Accessed 2024 October 9 https://www.canada.ca/en/health-canada/news/2023/09/health-canada-author...
-
- U.S. Food and Drug Administration. FDA takes action on updated mRNA COVID-19 vaccines to better protect against currently circulating variants. Accessed 2024 October 9 https://www.fda.gov/news-events/press-announcements/fda-takes-action-upd...
-
- Ma KC, Surie D, Lauring AS. Effectiveness of updated 2023–2024 (monovalent XBB.1.5) COVID-19 vaccination against SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineage hospitalization and a comparison of clinical severity—IVY Network, 26 hospitals, October 18, 2023–March 9, 2024. Clin Infect Dis. 2024. doi: ciae405 - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

