Toxic microbiome and progression of chronic kidney disease: insights from a longitudinal CKD-Microbiome Study
- PMID: 40461059
- PMCID: PMC12505076
- DOI: 10.1136/gutjnl-2024-334634
Toxic microbiome and progression of chronic kidney disease: insights from a longitudinal CKD-Microbiome Study
Abstract
Background: The gut microbiota has been linked to non-communicable diseases, including chronic kidney disease (CKD). However, the relationships between gut microbiome composition changes, uraemic toxins (UTs) accumulation, and diet on CKD severity and progression remain underexplored.
Objective: To characterise relationships between gut microbiome composition and functionality, UTs diet, and CKD severity and progression, as well as assess microbial contributions to UTs accumulation through mice faecal microbiota transplantation (FMT).
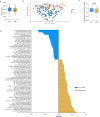
Design: This study profiled the gut microbiome of 240 non-dialysis patients with CKD (CKD-REIN cohort) using shotgun metagenomics, with follow-up in 103 patients after 3 years, with comparisons with healthy volunteers from the Milieu Intérieur cohort. A multiomics approach identifies features associated with CKD severity (and progression), with validation in an independent Belgian cohort. Experimental models used FMT to test CKD gut microbiome effects on UTs and kidney fibrosis. Changes in gut microbiome over time were evaluated, and the impact of diet on these changes was assessed.
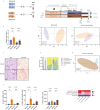
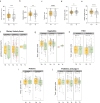
Results: Compared with matched healthy controls, patients with CKD exhibited gut microbiota alteration, with enrichment of UT precursor-producing species. Patients with severe CKD exhibited higher UT levels and greater enrichment of UT (precursor)-producing species in the microbiota than patients with moderate CKD. Over time, UT (precursor)-producing species increased, and a plant-based low protein diet appeared to mitigate these changes. FMT from patients with CKD to antibiotic-treated CKD model mice increased serum UT levels and exacerbated kidney fibrosis.
Conclusions: This study highlights the role of the microbiome and UTs in CKD, suggesting a potential therapeutic target to slow disease progression.
Keywords: EPIDEMIOLOGY; MICROBIOME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: CKD-REIN is supported by a public-private partnership with funding from 10 pharmaceutical companies as listed below. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. LK has received grants from Fresenius Kabi, Nestlé, Lallemand, AstraZeneca and consultancy or speaker fees or travel support from Astrazeneca, Lilly, Baxter, Bayer, and Fresenus Kabi. ZM received honoraria for lectures, educational events from AstraZeneca, GSK, Boehringer.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
