Efficacy and safety of acalabrutinib with best supportive care versus best supportive care in patients with COVID-19 requiring hospitalization
- PMID: 40461100
- PMCID: PMC12133263
- DOI: 10.1093/immhor/vlaf023
Efficacy and safety of acalabrutinib with best supportive care versus best supportive care in patients with COVID-19 requiring hospitalization
Abstract
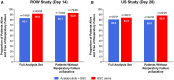
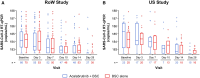
The efficacy and safety of acalabrutinib, a Bruton tyrosine kinase (BTK) inhibitor, was evaluated in 2 phase 2 studies in hospitalized patients with coronavirus disease 2019 (COVID-19) who received acalabrutinib + best supportive care (BSC) versus BSC alone (Clinicaltrials.gov: NCT04380688 and NCT04346199). The primary endpoint was the percentage of patients alive and free of respiratory failure on day 14 (rest of the world [RoW] study) and day 28 (US study). In the RoW study, 177 patients were randomized (acalabrutinib + BSC: n = 89; BSC: n = 88); in the US study, 62 patients were randomized (acalabrutinib + BSC: n = 31; BSC: n = 31). The percentage of patients who met the primary endpoint was similar in both studies (RoW study: acalabrutinib + BSC: 83.1%, BSC: 90.9%; US study: acalabrutinib + BSC: 80.6%, BSC: 83.9%). No new safety concerns were reported. Overall, no significant clinical benefit of adding acalabrutinib to BSC in patients hospitalized with COVID-19 was observed.
Keywords: COVID-19; acalabrutinib; best supportive care; bruton tyrosine kinase inhibitor; hospitalized patients.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
M.R.K. has none to disclose.
P.A.R. has none to disclose.
A.G. has none to disclose.
V.A.H.S. has none to disclose.
B.A.L.F. has none to disclose.
H.W.P. has none to disclose.
J.R.C. has none to disclose.
L.F. has none to disclose.
B.R.M. has none to disclose.
D.J.P. has served at a speaker’s bureau for AstraZeneca and CARIS and was provided research funding from Genentech/Roche, BeiGene, Gilead, Novartis, Astellas, Merck, LaNova, BMS, Agenus, Panbela, AstraZeneca, Mirati, and Lisata.
A.G. is an employee and shareholder of AstraZeneca.
P.P. and D.T. were employees and shareholders of AstraZeneca at the time that the study was conducted.
L.Z. was an employee and shareholder of AstraZeneca and stockholder of Gilead Sciences at the time that the study was conducted.
V.M. is an employee and shareholder of AstraZeneca; a family member is an employee and stockholder of Gilead Sciences.
P.S. reports receiving consulting fees or honoraria for lectures, presentations, speakers’ bureaus, and advisory boards from AstraZeneca, Alexion, Novartis, Pfizer, and Roche.
Figures




References
-
- World Health Organization. COVID-19 epidemiological update. World Health Organization; 2024.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

