Myoglobin expression improves T-cell metabolism and antitumor effector function
- PMID: 40461159
- PMCID: PMC12142173
- DOI: 10.1136/jitc-2025-011503
Myoglobin expression improves T-cell metabolism and antitumor effector function
Abstract
Background: The tumor microenvironment is frequently hypoxic and characterized by a scarcity of nutritional resources including a shortage of glucose. As effector T cells have high energy demands, tumor metabolism can contribute to T-cell dysfunction and exhaustion.
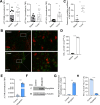
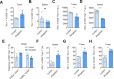
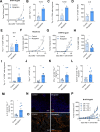
Methods: In this study, we determined hypoxia in spleen and tumor tissue from tumor-bearing C57BL/6J mice using reverse transcription polymerase chain reaction (RT-PCR), histology and flow cytometry. Next, CD8+ T cells isolated from C57BL6J mice or P14+ mice were transduced with Thy1.1 (Control) or Thy1.1-Myoglobin (Mb) packaged retrovirus. Expression of Mb was confirmed with RT-PCR and western blot. Cellular metabolism was determined by flow cytometry, transmission electron microscopy, focused ion beam scanning electron microscopy, Seahorse, metabolomics and luminescence assays. Mb expressing or control P14+ or OT-I+ T cells were transferred in B16F10-gp33 or MC38-ova tumor-bearing mice respectively and analyzed using flow cytometry and histology. B16F10-gp33 tumor-bearing mice were additionally treated with anti-programmed cell death protein-1 (PD-1) checkpoint inhibitor.
Results: Here we demonstrate that expression of the oxygen-binding protein myoglobin in T cells can boost their mitochondrial and glycolytic metabolic functions. Metabolites and tricarboxylic acid compounds were highly increased in the presence of myoglobin (Mb), which was associated with increased ATP levels. Mb-expressing T cells exhibited low expression of hypoxia-inducible factor-1α after activation and during infiltration into the tumor microenvironment (TME). Accordingly, Mb expression increased effector T-cell function against tumor cells in vitro with concomitant reductions in superoxide levels. Following adoptive transfer into tumor-bearing mice, Mb expression facilitated increased infiltration into the TME. Although T cells expressing Mb exhibited increased expression of effector cytokines, PD-1 was still detected and targetable by anti-PD-1 monoclonal antibodies, which in combination with transfer of Mb-expressing T cells demonstrated maximal efficacy in delaying tumor growth.
Conclusion: Taken together, we show that expression of Mb in T cells can increase their metabolism, infiltration into the tumor tissue, and effector function against cancer cells.
Keywords: Adoptive cell therapy - ACT; Colon Cancer; Immune Checkpoint Inhibitor.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JW, HCX, GT, LdSS and PAL are involved in the development of LCMV for clinical application in oncology in cooperation with or as founder of or as advisors to Abalos Therapeutics GmbH.
Figures



Similar articles
-
B and T lymphocyte attenuator (BTLA) and PD-1 pathway dual blockade promotes antitumor immune responses by reversing CD8+ T-cell exhaustion in non-small cell lung cancer.Front Immunol. 2025 May 20;16:1553042. doi: 10.3389/fimmu.2025.1553042. eCollection 2025. Front Immunol. 2025. PMID: 40463377 Free PMC article.
-
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice.Cancer Immunol Immunother. 2016 May;65(5):601-11. doi: 10.1007/s00262-016-1823-8. Epub 2016 Mar 24. Cancer Immunol Immunother. 2016. PMID: 27011014 Free PMC article.
-
Novel PD-1-targeted, activity-optimized IL-15 mutein SOT201 acting in cis provides antitumor activity superior to PD1-IL2v.J Immunother Cancer. 2025 Apr 17;13(4):e010736. doi: 10.1136/jitc-2024-010736. J Immunother Cancer. 2025. PMID: 40250867 Free PMC article.
-
Impacts of combining PD-L1 inhibitor and radiotherapy on the tumour immune microenvironment in a mouse model of esophageal squamous cell carcinoma.BMC Cancer. 2025 Mar 14;25(1):474. doi: 10.1186/s12885-025-13801-0. BMC Cancer. 2025. PMID: 40087599 Free PMC article.
-
CBX4 suppresses CD8+ T cell antitumor immunity by reprogramming glycolytic metabolism.Theranostics. 2024 Jun 17;14(10):3793-3809. doi: 10.7150/thno.95748. eCollection 2024. Theranostics. 2024. PMID: 38994031 Free PMC article.
Cited by
-
Mitochondrial Transfer Between Cancer and T Cells: Implications for Immune Evasion.Antioxidants (Basel). 2025 Aug 18;14(8):1008. doi: 10.3390/antiox14081008. Antioxidants (Basel). 2025. PMID: 40867904 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
