Digital health technologies in the accelerating medicines Partnership® Schizophrenia Program
- PMID: 40461469
- PMCID: PMC12134270
- DOI: 10.1038/s41537-025-00599-w
Digital health technologies in the accelerating medicines Partnership® Schizophrenia Program
Abstract
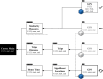
Although meta-analytic studies have shown that 25-33% of those at Clinical High Risk (CHR) for psychosis transition to a first episode of psychosis within three years, less is known about estimating the risk of transition at an individual level. Digital phenotyping offers a novel approach to explore the nature of CHR and may help to improve personalized risk prediction. Specifically, digital data enable detailed mapping of experiences, moods and behaviors during longer periods of time (e.g., weeks, months) and offer more insight into patterns over time at the individual level across their routine daily life. However, while novel digital health technologies open up many new avenues of research, they also come with specific challenges, including replicability of results and the adherence of participants. This paper outlines the design of the digital component of the Accelerating Medicines Partnership® Schizophrenia Program (AMP SCZ) project, a large international collaborative project that follows individuals at CHR for psychosis over a period of two years. The digital component comprises one-year smartphone-based digital phenotyping and actigraphy. Smartphone-based digital phenotyping includes 30-item short daily self-report surveys and voice diaries as well as passive data capture (geolocation, on/off screen state, and accelerometer). Actigraphy data are collected via an Axivity wristwatch. The aim of this paper is to describe the design and the three goals of the digital measures used in AMP SCZ to: (i) better understand the symptoms, real-life experiences, and behaviors of those at CHR for psychosis, (ii) improve the prediction of transition to psychosis and other health outcomes in this population based on digital phenotyping and, (iii) serve as an example for replicable and ethical research across geographically diverse regions and cultures. Accordingly, we describe the rationale, protocol and implementation of these digital components of the AMP SCZ project. **Link to video interview: https://vimeo.com/1060935583 *.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Celso Arango has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda. Dominic Dwyer has recieved honorary funds for one educational seminar for CSL Sequiris.John Kane has served as a consultant to or receives honoraria and/or travel support and/or speakers bureau: Alkermes, Allergan, Boehringer-Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HealthRhythms, HLS Therapeutics, Indivior, Intracellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, Karuna Therapeutics/Bristol Meyer-Squibb, LB Pharmaceuticals, Mapi, Maplight, Merck, Minerva, Neurocrine, Newron, Novartis, NW PharmaTech, Otsuka, Roche, Saladax, Sunovion, Teva. Paulo Fusar-Poli has received research funds or personal fees from Lundbeck, Angelini, Menarini, Sunovion, Boehringer Ingelheim, Proxymm Science, Otsuka, outside the current study Rachel Upthegove has received speaker fees at non promotional educational events: Otsuka: Consultancy for Viatris and Springer Healthcare. Honoary General Secretary British Association for Psychopharmacology (unpaid). Rene Kahn reports consulting: Alkermes, Boehringer-Ingelheim. John Torous reprots being an dvisor to Percison Mental Wellness. Research support from Otsuka. Joseph Kambeitz reports speaking or consulting fees from Janssen, Boehringer Ingelheim, ROVI and Lundbeck. Eric Yu Hai Chen report speaker fees at non-promotional educational events. Covadonga M. Diaz-Caneja has received grant support from Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation and honoraria or travel support from Angelini, Janssen, and Viatris. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures
References
-
- Catalan, A. et al. Annual Research Review: Prevention of psychosis in adolescents–systematic review and meta‐analysis of advances in detection, prognosis, and intervention. J. Child Psychol. Psychiatry62, 657–673 (2021). - PubMed
-
- Mei, C. et al. Preventive interventions for individuals at ultra-high risk for psychosis: an updated and extended meta-analysis. Clin. Psychol. Rev.86, 102005 (2021). - PubMed
-
- Addington, J. et al. Predictors of transition to psychosis in individuals at clinical high risk. Curr. Psychiatry Rep.21, 1–10 (2019). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

