Oncometabolite fumarate facilitates PD-L1 expression and immune evasion in clear cell renal cell carcinoma
- PMID: 40461489
- PMCID: PMC12134299
- DOI: 10.1038/s41419-025-07752-4
Oncometabolite fumarate facilitates PD-L1 expression and immune evasion in clear cell renal cell carcinoma
Abstract
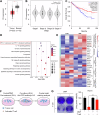
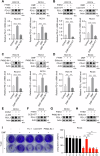
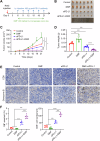
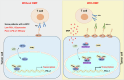
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of renal cell carcinoma (RCC), with a rising incidence worldwide. However, the mechanisms by which ccRCC evades immune surveillance remain incompletely understood. Our findings indicate that fumarate hydratase (FH) expression is significantly downregulated in ccRCC, resulting in fumarate accumulation, which is correlated with a poor prognosis in ccRCC patients. RNA sequencing analysis suggests that dimethyl fumarate (DMF), an FDA-approved fumarate analogue, may impact tumor immunity. Our further investigation reveals that both DMF and the FH inhibitor (FHIN1) can promote immune evasion in ccRCC by upregulating PD-L1. Pre-treatment of tumor cells with DMF notably inhibits the cytotoxic effect of T cells. Mechanistically, fumarate induces PD-L1 expression through succination of HIF-1α at C800, facilitating its interaction with importin α3, p300, and PKM2, which promotes HIF-1α nuclear localization and transcriptional activity. Moreover, combining DMF with PD-L1 blockade therapy significantly enhances the efficacy of immunotherapy and prolongs the survival of tumor-bearing mice. Taken together, our study elucidates a mechanism by which FH downregulation promotes immune evasion through the fumarate-HIF-1α/p300/PKM2-PD-L1 axis, providing a novel target, drug, and strategy to improve immunotherapy for ccRCC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

