Antigen persistence and TLR stimulation contribute to induction of a durable HIV-1-specific neutralizing antibody response
- PMID: 40461490
- PMCID: PMC12134134
- DOI: 10.1038/s41467-025-60481-2
Antigen persistence and TLR stimulation contribute to induction of a durable HIV-1-specific neutralizing antibody response
Abstract
HIV-1 Env glycoprotein (Env) immunogenicity is limited in part by structural instability and extensive glycan shielding and is likely the greatest obstacle to an HIV-1 vaccine. Stabilized Env trimers can elicit serum neutralizing antibodies, but the response is short-lived. Here we use Newcastle Disease Virus-like particle (NDV-VLP) platform to present stabilized versions of HIV-1 Env at high valency and in the context of varied conformational stability, adjuvants, dose, and antigen persistence. Influenza virus hemagglutinin, or SARS-CoV2 Spike-bearing VLPs rapidly induce neutralizing antibodies, in contrast, they were not induced by those bearing Env. A replicating adenovirus type 4 expressing Env rapidly induces autologous neutralizing antibodies. However, durable neutralizing antibodies are induced only when multiple features of a replicating virus infection are combined, with the largest impact from dose and escalating dose. In summary, we show here immunogenicity of HIV-1 Env could be improved by reproducing features of virus infection.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
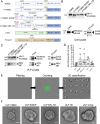
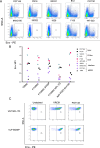
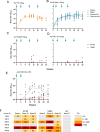
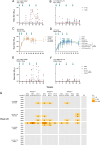


References
-
- Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med.357, 1903–1915 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

