Forebrain neural progenitors effectively integrate into host brain circuits and improve neural function after ischemic stroke
- PMID: 40461535
- PMCID: PMC12134216
- DOI: 10.1038/s41467-025-60187-5
Forebrain neural progenitors effectively integrate into host brain circuits and improve neural function after ischemic stroke
Abstract
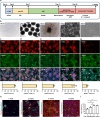
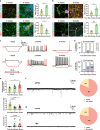
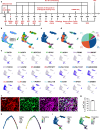
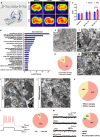
Human cortical neural progenitor cell transplantation holds significant potential in cortical stroke treatment by replacing lost cortical neurons and repairing damaged brain circuits. However, commonly utilized human cortical neural progenitors are limited in yield a substantial proportion of diverse cortical neurons and require an extended period to achieve functional maturation and synaptic integration, thereby potentially diminishing the optimal therapeutic benefits of cell transplantation for cortical stroke. Here, we generated forkhead box G1 (FOXG1)-positive forebrain progenitors from human inducible pluripotent stem cells, which can differentiate into diverse and balanced cortical neurons including upper- and deep-layer excitatory and inhibitory neurons, achieving early functional maturation simultaneously in vitro. Furthermore, these FOXG1 forebrain progenitor cells demonstrate robust cortical neuronal differentiation, rapid functional maturation and efficient synaptic integration after transplantation into the sensory cortex of stroke-injured adult rats. Notably, we have successfully utilized the non-invasive 18F-SynVesT-1 PET imaging technique to assess alterations in synapse count before and after transplantation therapy of FOXG1 progenitors in vivo. Moreover, the transplanted FOXG1 progenitors improve sensory and motor function recovery following stroke. These findings provide systematic and compelling evidence for the suitability of these FOXG1 progenitors for neuronal replacement in ischemic cortical stroke.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: Jing Fan and Anxin Wang are named inventors on two Chinese patents (Application Nos. CN 202110505838.6 and CN 201810298488.9) related to the neural induction and differentiation methodology (optimized RONA method) described in this study. These patents are owned by Hopstem Bioengineering Co., Ltd. and were granted in 2025 and 2020, respectively. All remaining authors declare no competing interests related to this work.
Figures
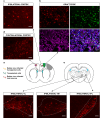
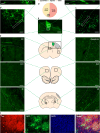
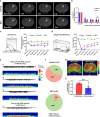
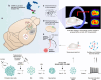
References
MeSH terms
Substances
Grants and funding
- 82030049, 32027802/National Natural Science Foundation of China (National Science Foundation of China)
- 82102095/National Natural Science Foundation of China (National Science Foundation of China)
- 82302262/National Natural Science Foundation of China (National Science Foundation of China)
- 82302267/National Natural Science Foundation of China (National Science Foundation of China)
- 82394433/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical

