The secretome of senescent monocytes predicts age-related clinical outcomes in humans
- PMID: 40461807
- PMCID: PMC12276915
- DOI: 10.1038/s43587-025-00877-3
The secretome of senescent monocytes predicts age-related clinical outcomes in humans
Abstract
Cellular senescence increases with age and contributes to age-related declines and pathologies. We identified circulating biomarkers of senescence and related them to clinical traits in humans to facilitate future noninvasive assessment of individual senescence burden, and efficacy testing of novel senotherapeutics. Using a nanoparticle-based proteomic workflow, we profiled the senescence-associated secretory phenotype (SASP) in THP-1 monocytes and examined these proteins in 1,060 plasma samples from the Baltimore Longitudinal Study of Aging. Machine-learning models trained on THP-1 monocyte SASP associated SASP signatures with several age-related phenotypes in a test cohort, including body fat composition, blood lipids, inflammatory markers and mobility-related traits, among others. Notably, a subset of SASP-based predictions, including a high-impact SASP panel, were validated in InCHIANTI, an independent aging cohort. These results demonstrate the clinical relevance of the circulating SASP and identify potential senescence biomarkers that could inform future clinical studies.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
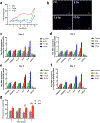





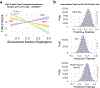
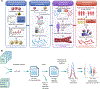
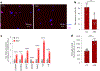




Update of
-
A plasma proteomic signature links secretome of senescent monocytes to aging- and obesity-related clinical outcomes in humans.medRxiv [Preprint]. 2024 Aug 3:2024.08.01.24311368. doi: 10.1101/2024.08.01.24311368. medRxiv. 2024. Update in: Nat Aging. 2025 Jul;5(7):1266-1279. doi: 10.1038/s43587-025-00877-3. PMID: 39371126 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

