Anti-sporozoite monoclonal antibody for malaria prevention: secondary efficacy outcome of a phase 2 randomized trial
- PMID: 40461818
- PMCID: PMC12353790
- DOI: 10.1038/s41591-025-03739-y
Anti-sporozoite monoclonal antibody for malaria prevention: secondary efficacy outcome of a phase 2 randomized trial
Abstract
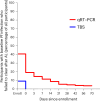
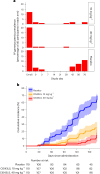
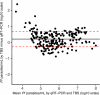
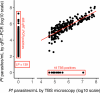
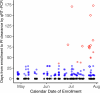
CIS43LS is a long-acting monoclonal antibody specific for the Plasmodium falciparum circumsporozoite protein expressed on sporozoites. We previously reported that CIS43LS is protective against P. falciparum infection as detected by thick blood smear (TBS; primary endpoint) in a phase 2 double-blind randomized trial involving 330 healthy Malian adults receiving placebo or a single intravenous infusion of 10 mg kg-1 or 40 mg kg-1 of CIS43LS (1:1:1). At enrollment, all participants received artemether-lumefantrine to clear possible P. falciparum infection. Although TBS examination is the standard assay to assess efficacy in malaria vaccines trials in endemic areas, it has poor analytical sensitivity; therefore, it remained unknown whether CIS43LS had achieved sterile protection against infection. Here we report the prespecified secondary efficacy endpoint that used a Plasmodium 18S rRNA quantitative reverse transcription-PCR (qRT-PCR) assay that is ~2,000-fold more sensitive than TBS. We analyzed 5,015 dried blood spots collected before CIS43LS or placebo administration and biweekly thereafter over a 6-month malaria season. At 6 months, efficacy of CIS43LS against qRT-PCR-detected infection assessed in a time-to-event analysis was 87.4% for 40 mg kg-1 (adjusted 95% confidence interval (CI), 79.5-92.3; P < 0.001) and 77.0% for 10 mg kg-1 (adjusted 95% CI, 65.0-84.0; P < 0.001) versus placebo. A post hoc analysis with a gametocyte mRNA-specific qRT-PCR assay showed 6-month efficacy against gametocytemia of 87.7% for 40 mg kg-1 (adjusted 95% CI, 75.6-93.8; P < 0.001) and 73.0% for 10 mg kg-1 (adjusted 95% CI, 54.0-84.0; P < 0.001), versus placebo. These data indicate that a single dose of anti-sporozoite monoclonal antibodies can achieve durable, sterile protection against P. falciparum infection, underscoring their potential to reduce malaria disease burden and transmission. ClinicalTrials.gov identifier: NCT04329104 .
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: R.A.S. has filed patent PCT/US2018/017826 describing the CIS43 antibody. The other authors declare no competing interests.
Figures


References
-
- World Malaria Report 2024 (World Health Organization, 2024); www.who.int/teams/global-malaria-programme/reports/world-malaria-report-...
-
- Dame, J. B. et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science225, 593–599 (1984). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

