A common computational and neural anomaly across mouse models of autism
- PMID: 40461847
- PMCID: PMC12403184
- DOI: 10.1038/s41593-025-01965-8
A common computational and neural anomaly across mouse models of autism
Abstract
Computational psychiatry studies suggest that individuals with autism spectrum disorder (ASD) inflexibly update their expectations. Here we leveraged high-yield rodent psychophysics, extensive behavioral modeling and brain-wide single-cell extracellular recordings to assess whether mice with different genetic perturbations associated with ASD show this same computational anomaly, and if so, what neurophysiological features are shared across genotypes. Mice harboring mutations in Fmr1, Cntnap2 or Shank3B show a blunted update of priors during decision-making. Compared with mice that flexibly updated their priors, inflexible updating of priors was associated with a shift in the weighting of prior encoding from sensory to frontal cortices. Furthermore, frontal areas in mouse models of ASD showed more units encoding deviations from the animals' long-run prior, and sensory responses did not differentiate between expected and unexpected observations. These findings suggest that distinct genetic instantiations of ASD may yield common neurophysiological and behavioral phenotypes.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

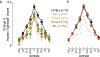
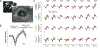
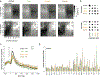
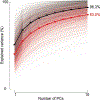
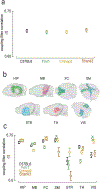
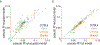

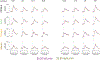
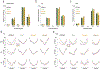





Update of
-
A common computational and neural anomaly across mouse models of autism.bioRxiv [Preprint]. 2024 May 8:2024.05.08.593232. doi: 10.1101/2024.05.08.593232. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Jul;28(7):1519-1532. doi: 10.1038/s41593-025-01965-8. PMID: 38766250 Free PMC article. Updated. Preprint.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (American Psychiatric Association, 2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

