Concomitant anti-CGRP and immunomodulatory treatments in patients with migraine: towards integrated management strategies
- PMID: 40461909
- PMCID: PMC12134006
- DOI: 10.1007/s00415-025-13177-y
Concomitant anti-CGRP and immunomodulatory treatments in patients with migraine: towards integrated management strategies
Abstract
Background: Preclinical evidence supports the immunoregulatory role of calcitonin gene-related peptide (CGRP) in migraine pathophysiology. The increasing use of anti-CGRP therapies in patients with migraine and other comorbidities raises the question whether the potential use of anti-CGRP monoclonal antibodies (CGRP-mAbs) therapies in combination with other immunological therapies is effective and safe.
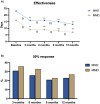
Methods: This multicenter study included patients with migraine receiving CGRP-mAbs combined with immunosuppressive and immunomodulatory treatments. Clinical and demographic data, treatment history, laboratory markers and treatment-emergent adverse events (TEAEs) were analyzed. Effectiveness outcomes included the change in monthly migraine days (MMD) and monthly headache days (MHD) at 3, 6, 9 and 12 months, alongside the > 50% response rate. Moreover, autoimmune disease progression was also evaluated. We explored differences between patients with and without autoimmune disease activation.
Results: Among 89 patients, there were 80 (90%) females with a mean age of 50 years (SD: 11), who had a high prevalence of psychiatric comorbidities (anxiety 44%, depression 49%) and medication overuse (68%). Patients receiving immunological treatments experienced significant reductions in MMD and MHD, with MMD decreasing from 16 (SD: 7) at baseline to 9 (SD: 8) at 6 months, and MHD dropping from 23 (SD: 8) to 17 (SD: 11). A 50% response in MMD was achieved by 46% at 6 months. TEAEs were reported in 28%, most commonly constipation (16%) and dizziness (9%).
Conclusions: CGRP-mAbs therapies combined with immunological treatments appear effective and safe in patients with autoimmune diseases. Larger prospective studies are necessary to confirm these findings and optimize management strategies.
Keywords: CGRP; Combination therapy; Immunity; Immunosuppression; Monoclonal antibodies; Neuroinflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare that they have no competing interest regarding the present manuscript. EC has received honoraria from Novartis, Chiesi, Lundbeck, MedScape, Lilly, TEVA, Dr Reddy’s; his salary has been partially funded by Río Hortega grant Acción Estratégica en Salud 2017–2020 from Instituto de Salud Carlos III (CM20/00217) and Juan Rodés fellowship, Subprograma Estatal de Incorporación de la Acción Estratégica en Salud 2023 (JR23/00065). He is a junior editor for Cephalalgia. AJ has received honoraria from Lilly, TEVA, Lundbeck and Abbvie. AMV has received honoraria for advisory board participation, consultancy, scientific communications, research support, and funding for travel and congress attendance from Teva, Lilly, Roche, UCB, Bial, Chiesi, Abbvie, Esai, Zambon, Kern Pharma, Pfizer, Janssen, Biogen Idec, BMS, Novartis, TEVA, Merck, Neuraxpharm, Genzyme, Sanofi, Bayer, Almirall and/or Celgene. A. S–S has received fees from TEVA for sponsored lectures. ABG-V has received speaker honoraria and/or clinical advisor from Novartis, Lilly, TEVA, Exeltis, Chiesi, Abbvie, Pfizer, Dr. Reddy´s and Lundbeck. SC received honoraria for participating on advisory boards and for collaborations as consultants and scientific communications; they also received research support as well as funding for travel and congress-attending expenses from Teva, Lilly, Roche, UCB, Kern Pharma, Pfizer, Biogen Idec, Novartis, TEVA, Merck, Genzyme, Sanofi, Bayer, Almirall, and Celgene. M-L. C has been involved as a consultant or lecturer for Novartis, Lundbeck and Teva. AL. GP has participated in Advisory Boards: Abbvie, Elly Lilly, Lundbeck, Organon, Pfizer, TEVA. Speaker boards: Abbvie, Elly Lilly, Exeltis, Lundbeck, TEVA. MHV received honoraria for participating on advisory boards and for collaborations as consultant, scientific communications, speaker, research support as well as funding for travel and congress attending expenses from AbbVie, Novartis, Lilly, TEVA, Lundbeck, Dr Reddy's, Pfizer, Almirall, Chiesi, Esai, Kern Pharma and Zambon. PP-R has received, in the last 3 years, honoraria as a consultant and speaker from AbbVie, Amgen, Dr Reddy’s, Eli Lilly, Lundbeck, Medscape, Novartis, Organon, Pfizer and Teva Pharmaceuticals. Her research group has received research grants from AbbVie, AGAUR, EraNet Neuron, FEDER RIS3CAT, Instituto Investigación Carlos III, MICINN, Novartis, and Teva Pharmaceuticals, and has received funding for clinical trials from AbbVie, Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis, Pfizer and Teva Pharmaceuticals. She is the Honorary Secretary of the International Headache Society, is an associate editor for Cephalalgia and Neurologia. She is a member of the Clinical Trials Guidelines Committee of the International Headache Society. PJG reports, over the last 36 months, grants from Kallyope, and personal fees from Aeon Biopharma, Abbvie, Amgen, Aurene, CoolTech LLC, Eli-Lilly and Company, Linpharma, Lundbeck, Pfizer, PureTech Health LLC, Satsuma, Shiratronics, Teva Pharmaceuticals, Tremeau, and Vial; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners and Vector Metric; fees for educational materials from CME Outfitters and WebMD; publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UptoDate and Wolters Kluwer; and a patent magnetic stimulation for headache (No. WO2016090333 A1) assigned to eNeura without fee. A.G.M has received speaker honoraria from TEVA, Lilly and Altermedica. Her salary has been partially funded by Río Hortega grant Acción Estratégica en Salud from Instituto de Salud Carlos III (CM21/00178) and Juan Rodés fellowship, Subprograma Estatal de Incorporación de la Acción Estratégica en Salud 2023 (JR23/00005). She is the principal investigator of the Research Project funded by Instituto de Salud Carlos III (grant number PI24/01085) and co-funded by FEDER and FSE. She is board member of the Future Headache Experts of the European Headache Federation, secretary of the Residents and Research Fellows (RRFS) of the European Academy of Neurology (EAN) and coordinator of the junior Headache Group of the Spanish Society of Neurology (jGECSEN). Consent for publication: Consent for publication was obtained from patients participating in the present study. Details that might disclose the identity of the subjects under study should be omitted. Ethics approval and consent to participate: Ethics approval and consent to participate was obtained from the institutional ethics committee of Hospital Universitario de la Princesa (Number: 4563) and the project has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Figures
Similar articles
-
Patient satisfaction with calcitonin gene-related peptide monoclonal antibodies in migraine: A multicenter prospective cohort study.Headache. 2025 Jun;65(6):994-1004. doi: 10.1111/head.14913. Epub 2025 Mar 26. Headache. 2025. PMID: 40135519
-
Long-term safety and tolerability of zavegepant 10-mg nasal spray with concomitant use of anti-calcitonin gene-related peptide monoclonal antibodies or other select preventive migraine medications: Results from a phase 2/3 open-label study.Headache. 2025 Jul-Aug;65(7):1180-1189. doi: 10.1111/head.14954. Epub 2025 May 20. Headache. 2025. PMID: 40391567 Clinical Trial.
-
Preventive drug treatments for adults with chronic migraine: a systematic review with economic modelling.Health Technol Assess. 2024 Oct;28(63):1-329. doi: 10.3310/AYWA5297. Health Technol Assess. 2024. PMID: 39365169 Free PMC article.
-
Persistent effectiveness of CGRP antibody therapy in migraine and comorbid medication overuse or medication overuse headache - a retrospective real-world analysis.J Headache Pain. 2024 Jul 4;25(1):109. doi: 10.1186/s10194-024-01813-3. J Headache Pain. 2024. PMID: 38965463 Free PMC article.
-
Treatment of resistant chronic migraine with anti-CGRP monoclonal antibodies: a systematic review.Eur J Med Res. 2022 Jun 4;27(1):86. doi: 10.1186/s40001-022-00716-w. Eur J Med Res. 2022. PMID: 35659086 Free PMC article.
References
-
- Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A et al (2021) Migraine: epidemiology and systems of care. Lancet 397(10283):1485–1495 - PubMed
-
- Burch RC, Buse DC, Lipton RB (2019) Migraine: epidemiology, burden, and comorbidity. Neurol Clin 37(4):631–649 - PubMed
-
- Steiner TJ, Stovner LJ (2023) Global epidemiology of migraine and its implications for public health and health policy. Nat Rev Neurol 19(2):109–117 - PubMed
-
- Mohammadi M, Kankam SB, Salehi S, Mohamadi M, Mohammadi A, Firoozabadi SRD et al (2023) The association between multiple sclerosis and migraine: a meta-analysis. Mult Scler Relat Disord. 79:104954 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

