Population Pharmacokinetic Analysis of Enalapril and Enalaprilat in Newly Treated Children with Heart Failure: Implications for Safe Dosing of Enalapril (LENA Studies)
- PMID: 40461938
- PMCID: PMC12185616
- DOI: 10.1007/s40262-025-01520-5
Population Pharmacokinetic Analysis of Enalapril and Enalaprilat in Newly Treated Children with Heart Failure: Implications for Safe Dosing of Enalapril (LENA Studies)
Abstract
Background: Enalapril orodispersible minitablets (ODMT) have been authorised by the European Medicines Agency for the treatment of heart failure in children from birth to 17 years of age in 2023. Consequently, the use of enalapril in very young and angiotensin-converting enzyme inhibitor (ACEi) naïve patients is expected to increase.
Objectives: Simultaneous characterisation of the pharmacokinetics (PK) of enalapril and the active metabolite enalaprilat in ACEi naïve children with heart failure using a combined population pharmacokinetic (PopPK) model and identification of clinically relevant covariates for the dosing of enalapril in this population.
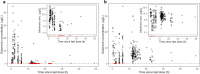
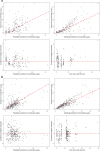
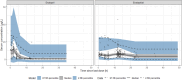
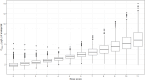
Methods: Data of ACEi naïve subjects from the European project 'Labeling of Enalapril from Neonates up to Adolescents' (LENA) were analysed using nonlinear mixed effects modelling. In the prospective, open-label, multicentre phase II/III PK bridging studies, children with heart failure due to dilated cardiomyopathy (DCM) and congenital heart disease (CHD) received enalapril ODMT according to an age- and weight-dependent dosing regimen. Allometric scaling was implemented for the disposition parameters of enalapril and enalaprilat. Stepwise covariate modelling was used to test the covariates age, sex, serum creatinine and Ross score. The final model was validated using nonparametric bootstrap analysis. Simulations were performed to assess the impact of the covariates after the first dose and at steady state.
Results: The analysed dataset comprised 173 enalapril and 268 enalaprilat serum concentrations from 34 subjects aged 25 days to 2.1 years (median age = 0.3 years). A combined model consisting of a one-compartment model for enalapril coupled with a one-compartment model for enalaprilat with absorption lag was selected as the structural model. Covariate analysis revealed that the weight-adjusted apparent clearance of enalaprilat increases with increasing age and decreases with increasing serum creatinine. In addition, the weight-adjusted apparent volume of distribution of enalaprilat decreases with increasing Ross score. The simulations indicated that serum creatinine levels above the normal reference range, age and weight were clinically relevant covariates for both the first dose and the steady state dose of enalapril. Furthermore, the simulations indicated that the Ross score is a clinically relevant covariate for the first dose of enalapril.
Conclusions: The results of the PopPK analysis and simulations indicated that, in addition to the currently considered parameters of weight and renal function, the parameters of age and severity of heart failure should also be considered when dosing enalapril in children with heart failure.
Trial registration: Trial registration number (date of registration): EudraCT 2015-002335-17 (30 November 2015), EudraCT 2015-002396-18 (30 November 2015). The trials were registered on the EU Clinical Trials Register ( https://www.clinicaltrialsregister.eu ).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open Access funding enabled and organized by Projekt DEAL. The research work is based on the data of the project Labeling of Enalapril from Neonates up to Adolescents (LENA), which was funded by a European Union Seventh Framework Program (FP7/2007-2013) under the grant agreement no. 602295. The open access fee was funded by the Heinrich Heine University Düsseldorf. Conflicts of Interest: The authors declare no conflicts of interest. Availability of Data and Material:: The analysed datasets from the LENA project are not publicly available due to the data protection contract of the product owner. Ethics Approval: The study protocols responding to specific national requirements were submitted to the responsible Independent Ethics Committees (IECs) in the participating countries for review and received approval. The address from the Ethics Committee of the coordinating principal investigator’s IEC of Study EudraCT 2015-002335-17 (DCM patients) was: Secretariaat Medisch Ethische Toetsings Commissie Erasmus MC, Postbus 2040, 3000 CA Rotterdam, the Netherlands, NL, dossiers: 4 December 2015, nos. NL54914.078.15, NL54738.078.15, MEC-2015-634 and MEC-2015-635; Ethics Committee UMC Utrecht, NL: 17 February 2016 nos. Mvd/vb/16/004864, Mvd/vb/16/004964; Medical Research Council, Ethics Committee for Clinical Pharmacology, National Institute of Pharmacy and Nutrition, Budapest, 30 November 2015, nos. OGYI/36681-7/2015 and OGYI/36999-9/2015; Ethikkommission Medizinische Universität Wien: 21 December 2015 no.1803/2015. The address of the coordinating principal investigator’s IEC of study EudraCT 2015-002396-18 (CHD patients) was: Ethics Committee of the University Children’s Hospital in Belgrade and the Institute of Mother and Child Health ‘Dr Vukan Čupić’ Univerzitetska Dečja Klinika, Tirsova 10, 11129 Belgrade, Serbia. 29 February 2016 and 5 April 2017, nos. 26/307 and 8/9; Ethikkommisison der Ärztekammer Hamburg, Germany, 22 May 2017, PVN9495 and PVN5496. The studies were conducted in accordance with the Declaration of Helsinki. Consent to Participate: Informed parental consent was obtained for each subject included in the studies. Assent of participating children was obtained in accordance with national requirements. Consent for Publication: Not applicable. Code Availability: Not applicable. Author Contributions: Conception and design of the work were developed by M.S., C.W. and S.L. Data preparation was performed by M.S. M.S. performed the population pharmacokinetic analysis and simulations. W.C. and S.L. critically reviewed and discussed the analysis and simulations. M.S. drafted the manuscript. W.C. and S.L. critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Figures


References
-
- EMA. Aqumeldi (enalapril): European public assessment report [online]. Available from URL: https://www.ema.europa.eu/en/documents/overview/aqumeldi-epar-medicine-o.... Accessed 9 Aug 2024.
-
- Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: executive summary. Corrected. J Heart Lung Transplant. 2014;33(9):888–909. - PubMed
-
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

