Efficacy and safety of venetoclax and azacitidine for acute myeloid leukemia in China: a real-world single-center study
- PMID: 40461963
- PMCID: PMC12135334
- DOI: 10.1186/s12885-025-14167-z
Efficacy and safety of venetoclax and azacitidine for acute myeloid leukemia in China: a real-world single-center study
Abstract
Background: Venetoclax (VEN) and azacitidine (AZA) are used to treat patients with newly diagnosed acute myeloid leukaemia (AML) who are unfit for intensive chemotherapy and those with relapsed or refractory AML. Understanding the real-world usage patterns and outcomes after VEN-AZA therapy failure is crucial, yet poorly studied.
Methods: This single-centre retrospective cohort study included 50 patients with AML (29 newly diagnosed and 21 relapsed or refractory cases) who were treated between January 2020 and November 2023. The primary endpoint was overall survival (OS), and secondary endpoints included composite complete remission (CR), partial remission (PR), overall response rate (ORR), event-free survival (EFS), minimal residual disease (MRD), adverse events (AEs), and post-VEN-AZA failure.
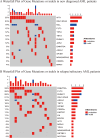
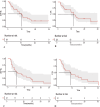
Results: Among the newly diagnosed patients (median age, 74 years; follow-up 10.1 months), the median EFS was 9.87 months (95% CI, 6.54-13.2 months) and OS was 11.93 months (95% CI, 7.6-16.29 months). The ORR was 85.7%, CR/CR with incomplete haematologic recovery (CRi) was achieved in 67.9% of patients, and MRD negativity was observed in 26.3% of the cohort. Post-treatment failure included VEN-AZA combined with gilteritinib, chidamide, or selinexor, which resulted in PR or CRi. The median OS after post-failure was 1.6 months. Among relapsed or refractory cases (median age 65 years; follow-up 8.53 months), median EFS was 5.2 months (95% CI, 1.8-8.6 months), and OS was 9.1 months (95% CI, 3.01-15.19 months). The ORR was 52.4%, CR/CRi was achieved in 42.9% of patients, and MRD negativity was observed in 11.11% of the cohort. Post-failure treatments include induction chemotherapy, VEN-AZA combined with enasidenib or gilteritinib, and participation in clinical trials, which yielded varying responses. The median OS after failure was 0.67 months, and the most common AEs were haematological and infectious complications.
Conclusions: VEN-AZA demonstrated high efficacy and manageable toxicity in patients with AML. Following VEN-AZA failure, the combination of VEN-AZA with targeted therapies has shown better efficacy than other VEN-AZA alone, whereas induction chemotherapy or clinical trials were preferred after second-line failure. Larger multicentre studies are warranted to validate these findings.
Keywords: Acute myeloid leukemia; Hypomethylating agents; Refractory; Relapse; Venetoclax.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This study was approved by the Ethics Committee of Beijing Hospital, National Center of Gerontology. The relevant reference number was 2023BJYYEC-224-0. all participants signed a written informed consent form in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- No.BJ-2024-191/the National High Level Hospital Clinical Research Funding
- No.BJ-2022-127/the National High Level Hospital Clinical Research Funding
- No.BJ-2023-078/the National High Level Hospital Clinical Research Funding
- No. 7232137/the Beijing Natural Science Foundation
- No. 7222158/the Beijing Natural Science Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials

