Effects of deprescribing antidepressants in nursing home residents with dementia-a cluster randomized controlled trial
- PMID: 40462023
- PMCID: PMC12131616
- DOI: 10.1186/s12875-025-02894-y
Effects of deprescribing antidepressants in nursing home residents with dementia-a cluster randomized controlled trial
Abstract
Background: Older nursing home residents with dementia are commonly prescribed antidepressants despite limited evidence of clinical effect and a high risk of side effects. Deprescribing can be challenging and is often not attempted. The aim of the study is to investigate the effect of a multifaceted intervention targeting nursing home general practitioners and their collaboration with the nursing home staff on the reduction of antidepressant medication in older nursing home residents with dementia.
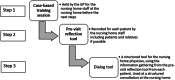
Method: The study is a cluster-randomized, non-blinded, controlled trial. General practitioners working as nursing home physicians in the Capital Region of Denmark were recruited between June 1 and October 1, 2021. Eligible participants were individuals with dementia (diagnosed or suspected), ≥ 72 years old, receiving one or more antidepressants, and living in a nursing home with the associated nursing home physician. The complex intervention consisted of three main parts: 1) a training session occurring in the nursing home, 2) a pre-visit reflection tool, and 3) a dialog tool used during a structured home visit at the nursing home. The control group received enhanced care as usual. Primary outcome was the reduction of the total defined daily dose of antidepressants from pre- to post-intervention in the intervention group, compared to the control group. Secondary outcomes included mortality, changes in other psychotropic medication, hospitalization, and symptoms changes.
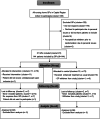
Results: We recruited 21 clusters with 128 eligible participants (62/66 in intervention and control). Four clusters withdrew. Most participants were women, and the median age was 85. They received an average of nine different drugs, and the most commonly prescribed antidepressants were sertraline and mirtazapine. The OR for the reduction of antidepressants in the intervention group versus control was 2.3 (95% CI = 0.84-6.2). Mortality rates were similar between groups.
Conclusions: The intervention did not significantly reduce antidepressant use among older nursing home residents with dementia. Further optimization and testing in a larger study are needed.
Trial registration: ClinicalTrials.gov ID NCT04985305, registration date: 2021-08-02.
Keywords: Antidepressants; Dementia; Deprescribing; General practice; Nursing home.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in compliance with the Helsinki Declaration in its latest form, good clinical practice guidelines, and followed the rules for informed consent. The Regional Ethical Committee was informed about the trial and waivered ethical approval according to Danish legislation (Journal no: H-20084023). The University of Copenhagen approved study procedures and informed consent was obtained verbally and recorded from all participants according to Danish legislation concerning non-intervention trials. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
A cluster-randomized trial of a complex intervention to encourage deprescribing antidepressants in nursing home residents with dementia: a study protocol.Trials. 2022 May 16;23(1):410. doi: 10.1186/s13063-022-06368-9. Trials. 2022. PMID: 35578351 Free PMC article.
-
Tailoring a complex intervention to reduce antidepressants in institutionalized older persons with dementia.BMC Health Serv Res. 2022 Dec 26;22(1):1582. doi: 10.1186/s12913-022-08961-9. BMC Health Serv Res. 2022. PMID: 36572903 Free PMC article. Clinical Trial.
-
Interventions for preventing and reducing the use of physical restraints for older people in all long-term care settings.Cochrane Database Syst Rev. 2023 Jul 28;7(7):CD007546. doi: 10.1002/14651858.CD007546.pub3. Cochrane Database Syst Rev. 2023. PMID: 37500094 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Algorithm-based pain management for people with dementia in nursing homes.Cochrane Database Syst Rev. 2022 Apr 1;4(4):CD013339. doi: 10.1002/14651858.CD013339.pub2. Cochrane Database Syst Rev. 2022. PMID: 35363380 Free PMC article.
References
-
- Lundby C, Jensen J, Larsen SP, Hoffmann H, Pottegård A, Reilev M. Use of medication among nursing home residents: a Danish drug utilisation study. Age Ageing. 2020;49(5):814–20. 10.1093/ageing/afaa029. - PubMed
-
- Sundhedsdatastyrelsen. eSundhed: Antipsykotika [antipsychotics]. 2022. https://www.esundhed.dk/Emner/Laegemidler/Antipsykotika. Accessed December 12. 2023.
-
- Kessing LV, Harhoff M, Andersen PK. Treatment with antidepressants in patients with dementia–a nationwide register-based study. Int Psychogeriatr. 2007;19(5):902–13. 10.1017/s1041610206004376. - PubMed
-
- Farina N, Morrell L, Banerjee S. What is the therapeutic value of antidepressants in dementia? A narrative review. Int J Geriatr Psychiatry. 2017;32(1):32–49. 10.1002/gps.4566. - PubMed
-
- Coupland CA, Dhiman P, Barton G, Morriss R, Arthur A, Sach T et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess. 2011;15(28):1–202, iii-iv. 10.3310/hta15280. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
