Revisiting the therapeutic landscape of tauopathies: assessing the current pipeline and clinical trials
- PMID: 40462159
- PMCID: PMC12135284
- DOI: 10.1186/s13195-025-01775-x
Revisiting the therapeutic landscape of tauopathies: assessing the current pipeline and clinical trials
Abstract
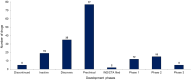
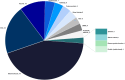
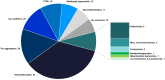
Microtubule associated protein tau (MAPT) is a naturally occurring protein that plays a significant role in stabilizing microtubules, which are essential for the transport of nutrients and other materials within neurons. In tauopathies, tau protein assembles into mis-folded multimers ranging from soluble oligomers to insoluble aggregates, known as neurofibrillary tangles, neuropil threads and are components of neuritic plaques. These abnormal tau assemblies collectively are thought to disrupt the normal function of neurons and lead to their death. Tauopathies are a leading cause of neurodegeneration, and there are no approved disease modifying therapies targeting the tau pathology for any tauopathy. This review is a two-year update to an initial review of preclinical, clinical, and recently discontinued therapeutic programs in development focused on ameliorating tau pathology. This review outlines the landscape of therapeutic drugs indexed through January 1, 2025. Currently, there are 170 drugs monitored in the pipeline, one less than in the previous period. In the clinic, there are five candidates in phase 3 trials, 15 in phase 2 trials, and 12 in phase 1 trials. In total, there are four less candidates in clinical trials during this review period than the last. New to this review is the inclusion of the clinical development of tau positron emission tomography (PET) ligands which undergo regulatory oversite. In addition to the one FDA-approved tau PET ligand Tauvid™ (flortaucipir), there are six additional tau PET ligands currently in active clinical trials.
Keywords: Alzheimer's disease; Argyrophilic grain disease; Corticobasal degeneration; Drug development; Frontotemporal dementia; MAPT; Pick’s disease; Positron emission tomography; Primary age-related tauopathy; Progressive supranuclear palsy; Tau; Tauopathy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Not applicable. Consent for publication: All authors have consented for this review paper to be published. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical