Targeting CD39 boosts PD-1 blockade antitumor therapeutic efficacy via strengthening CD8 + TILs function and recruiting B cells in cervical cancer
- PMID: 40462160
- PMCID: PMC12131488
- DOI: 10.1186/s12951-025-03500-0
Targeting CD39 boosts PD-1 blockade antitumor therapeutic efficacy via strengthening CD8 + TILs function and recruiting B cells in cervical cancer
Abstract
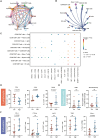
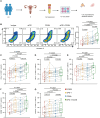
Although the programmed cell death protein 1 (PD-1) blockade has been authorized for the treatment of recurrent and metastatic cervical cancer (CC) patients, a significant proportion of CC patients show low objective response rates (ORR) to immune checkpoint blockades (ICBs). Therefore, identifying novel combination treatment strategies to enhance ICBs therapeutic efficacy for CC patients is urgently needed. Here, we discovered that CD39 was highly expressed in exhausted CD8 + T cells from 10 CC patients in our center via single-cell RNA sequencing (scRNA-seq). Furthermore, we validated that CC patients with CD39 highly expressed in CD8 + T cells associated with poor prognosis and immunoevasive subtype of CC both in cohort from our center and the Cancer Genome Atlas (TCGA) database. Moreover, it was also confirmed that CD39-inhibiting not only enhanced the cytotoxicity of CD8 + tumor-infiltrating lymphocytes (TILs) but also promoted the infiltration of B cells through increasing CXCL13 secretion both in vitro experiments and subcutaneous tumor models, thereby amplifying anti-tumor immunity of PD-1 blockade. What was more, we have developed a liposome containing POM-1, which effectively enhanced the anti-tumor effect of POM-1. Our findings provide compelling evidence that targeting CD39 represents a promising "two birds with one stone" strategy for cervical cancer treatment.
Keywords: B cells; Cervical cancer (CC); Exhausted T cells; Immune checkpoint blockage (ICB); Tumor microenvironment; αCD39.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and informed consent: This study received ethical approval from the Ethics Committee of the Fudan University Obstetrics and Gynecology Hospital (2023 − 107) and the Animal Ethics Committee of Fudan University (202312014 S). The study was conducted in accordance with the principles of the Declaration of Helsinki. All the patients involved in this study provided written informed consent for sample collection and data analysis. Consent for publication: All authors of this study agreed to publish. Competing interests: The authors declare no competing interests.
Figures







References
-
- Sung H, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Cervical Cancer Version 1.2025.
-
- Institute NC. Cancer STAT facts: cervix uteri cancer. Available from: https://seer.cancer.gov/statfacts/html/cervix.html
-
- Balar AV, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

