MiR221/222 in the conditioned medium of adipose-derived stem cells attenuates particulate matter and high-fat diet-induced cardiac apoptosis
- PMID: 40462170
- PMCID: PMC12135233
- DOI: 10.1186/s13287-025-04381-8
MiR221/222 in the conditioned medium of adipose-derived stem cells attenuates particulate matter and high-fat diet-induced cardiac apoptosis
Abstract
Background: Air pollution and obesity are crucial risk factors for cardiovascular disease (CVD), with epidemiological evidence indicating that air pollution exacerbates obesity-induced cardiac damage. Treatment with adipose-derived stem cells (ADSCs) attenuates cardiac damage by releasing paracrine factors. However, the effects of ADSCs on air pollution- and obesity-induced cardiomyocyte apoptosis and the related mechanisms are still unclear.
Methods: Palmitic acid (PA) and a high-fat diet (HFD) were used to cause obesity, and particulate matter (PM) was used to simulate air pollution in the study. We studied the impact of conditioned medium from adipose-derived stem cells (ADSC-CM) on the apoptosis of PA + PM-treated H9c2 cells and HFD + PM-treated mouse cardiomyocytes and the underlying mechanisms involved.
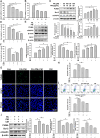
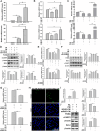
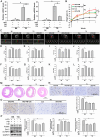
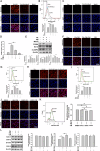
Results: The levels of apoptosis-related proteins (PUMA and cleaved caspase-3) were significantly increased in PA + PM-treated H9c2 cells and HFD + PM-treated mouse cardiomyocytes, whereas the antiapoptotic protein Bcl-2 expression was reduced. However, ADSC-CM treatment effectively reduced the PUMA and cleaved caspase-3 expression but increased the Bcl-2 expression. ADSC-CM significantly reduced PA + PM- and HFD + PM-induced cardiomyocyte apoptosis, as detected by the TUNEL assay. RT-qPCR revealed that PA + PM and HFD + PM significantly reduced miR221/222 levels, whereas ADSC-CM treatment increased miR221/222 levels. Furthermore, knockout (KO) and transgenic (TG) mice were used to demonstrate that miR221/222 in ADSC-CM ameliorated cardiac apoptosis that was induced by HFD + PM treatment. Furthermore, PA + PM treatment increased the reactive oxygen species (ROS) production, which triggered mitochondrial fission and contributed to apoptosis. However, ADSC-CM effectively reduced ROS levels and regulated mitochondrial fission, alleviating cellular apoptosis.
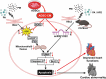
Conclusions: Our findings demonstrated that ADSC-CM attenuated PA + PM-induced cardiomyocyte apoptosis by modulating miR221/222 levels and suppressing ROS production.
Keywords: miR221/222; Conditioned medium from cultured adipose-derived stem cell (ADSC-CM); High-fat diet (HFD); Mitochondrial fission; Palmitic acid (PA); Particulate matter (PM); Reactive oxygen species (ROS).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The studies involving experimental animals were performed according to the guidelines of the Institutional Animal Care and Use Committee, National Taiwan University College of Medicine, and College of Laboratory Animal Center (Project title: To study the effects of particulate matter 2.5 and high fat on cardiac injury: the role of mitochondrial dynamics and mitophagy; Approval number: 20210334; Date of approval: Aug 1, 2022). ADSCs were purchased from LONZA (Basel, Switzerland), and the cells were used with permission for research applications. Consent for publication: Not applicable. Competing interests: The authors have no conflicts of interest.
Figures


References
-
- Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the american heart association. Circulation. 2017;136:e1–23. - PubMed
MeSH terms
Substances
Grants and funding
- MOST 108-2320-B-002-065-MY3/National Science and Technology Council
- NSTC 111-2320-B-002-022-MY3/National Science and Technology Council
- ZRRPF6N001/Chang Gung University of Science Foundation
- CORPF6P0041/Chang Gung Medical Research Program Foundation
- CORPF6P0042/Chang Gung Medical Research Program Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

