A Near-Infrared-II Luminescent and Photoactive Vanadium(II) Complex with a 760 ns Excited State Lifetime
- PMID: 40462271
- PMCID: PMC12186473
- DOI: 10.1021/jacs.5c04471
A Near-Infrared-II Luminescent and Photoactive Vanadium(II) Complex with a 760 ns Excited State Lifetime
Abstract
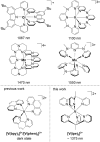
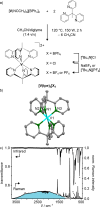
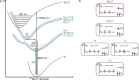
Ruthenium and iridium are key components in the most important applications of photoactive complexes, namely, light-emitting devices, photocatalysis, bioimaging, biosensing, and photodynamic therapy. Especially, near-infrared (NIR) emissive materials are required in fiber-optic telecommunications, anticounterfeit inks, night-vision readable displays, and bioimaging. Replacing rare and expensive precious metals with more abundant first-row transition metals is of great interest; however, photophysical properties and the chemical stability of 3d metal complexes are often insufficient. Here, we tackle these challenges with a nonprecious metal polypyridine vanadium(II) complex that shows emission above 1300 nm with excited state lifetimes of up to 760 ns. Strong light absorption in the visible spectral region and exceptional stability in the presence of oxygen enable photocatalysis in water and acetonitrile using green to orange-red light for excitation. This study unravels a new design principle for NIR-II luminescent and photoactive complexes based on the abundant first-row transition metal vanadium.
Figures



References
-
- Hockin B. M., Li C., Robertson N., Zysman-Colman E.. Photoredox catalysts based on earth-abundant metal complexes. Catal. Sci. Technol. 2019;9:889–915. doi: 10.1039/C8CY02336K. - DOI
-
- Dorn, M. ; East, N. R. ; Förster, C. ; Kitzmann, W. R. ; Moll, J. ; Reichenauer, F. ; Reuter, T. ; Stein, L. ; Heinze, K. . d-d and charge transfer photochemistry of 3d metal complexes. Comprehensive Inorganic Chemistry III, Elsevier, 2023, 707–788 10.1016/B978-0-12-823144-9.00063-7 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

