Antidepressant drugs promote the spread of broad-host-range plasmid in mouse and human gut microbiota
- PMID: 40462285
- PMCID: PMC12143698
- DOI: 10.1080/19490976.2025.2514138
Antidepressant drugs promote the spread of broad-host-range plasmid in mouse and human gut microbiota
Abstract
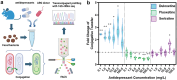
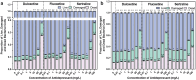
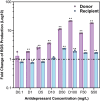
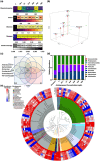
Antibiotic resistance is a global public health challenge. The gut microbiota serves as a reservoir for antibiotic resistance genes (ARGs), facilitating their transfer between bacteria. With the rising incidence of major depressive disorders (MDD), antidepressant prescriptions have surged. Previous pure-culture studies suggest that antidepressants exhibit antibiotic-like side effects, but their impact on gene transfer in microbial communities remains unclear. Here, we report that clinically relevant doses of antidepressants duloxetine and sertraline enhance the transfer of a broad-host range conjugative plasmid across bacterial phyla from mice gut microbiota. Tests in human gut simulators confirmed that duloxetine facilitates plasmid transfer in human gut microbiota. Mechanistic analyses revealed that antidepressants increase reactive oxygen species levels and alter bacterial cell membrane permeability. Using fluorescence-activated cell sorting and 16S rRNA gene sequencing, we discovered that antidepressants alter plasmid transfer patterns at both phylum and genus levels, driving ARG exchange among opportunistic pathogens. Our findings suggest that antidepressant use may promote the spread of antibiotic resistance between commensal and pathogenic bacteria, raising important public health concerns.
Keywords: Antidepressants; antibiotic resistance; conjugation; gut microbiota; horizontal gene transfer; plasmid.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
