Analytical treatment interruption among women with HIV in southern Africa who received VRC01 or placebo in the Antibody Mediated Prevention Study: ATI stakeholder engagement, implementation and early clinical data
- PMID: 40462491
- PMCID: PMC12134397
- DOI: 10.1002/jia2.26495
Analytical treatment interruption among women with HIV in southern Africa who received VRC01 or placebo in the Antibody Mediated Prevention Study: ATI stakeholder engagement, implementation and early clinical data
Abstract
Introduction: Antiretroviral therapy (ART) prevents and treats, but does not eradicate, HIV. Early ART initiation is associated with post-ART virologic control, particularly among African women, and anti-HIV-1 broadly neutralizing antibodies (bnAbs) may modulate immune responses to HIV. We evaluate whether early ART with or without anti-HIV-1 bnAb VRC01, present at HIV acquisition, is associated with later ART-free control in African women and we assess potential associations with observed control.
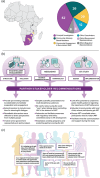
Methods: Stakeholder engagement informed analytical treatment interruption (ATI) study design and implementation. Participants who received placebo or VRC01 and acquired HIV in the Antibody Mediated Prevention efficacy trial were assessed for ATI eligibility, including HIV acquisition within 8 weeks of receiving VRC01 or placebo, followed by early ART initiation and ≥1 year of viral suppression. Participation facilitators and barriers were assessed. From May 2021 to February 2024, participants enrolled, stopped ART and received frequent viral load and CD4+ T-cell count monitoring for safety and assessment of meeting ART reinitiation criteria.
Results: Thirteen women enrolled from southern Africa. No ATI-related serious adverse events (AEs), HIV transmissions, pregnancies or ≥Grade 2 AEs were observed. Eight sexually transmitted infections were diagnosed in seven women during ATI. Two participants had tenofovir levels consistent with use during ATI; 2/11 (18%) who completed ATI without antiretroviral use exhibited ART-free control for ≥32 weeks. The median time to confirmed VL≥200 was 5.4 weeks (range 2.7-112). The most common ART reinitiation criterion met was virologic (n = 7). VRC01 receipt proximate to HIV acquisition was not associated with control. Controllers versus non-controllers did not differ by early post-acquisition viral load kinetics, acquired virus characteristics, or time from estimated acquisition to closest infusion or to ART initiation.
Conclusions: In a safe, well-tolerated ATI, 18% of 11 African women exhibited post-intervention control. Design and implementation lessons inform future ATIs in Africa. Analyses of peri-acquisition and post-ATI host and viral characteristics can inform the development of interventions for HIV cure, prevention and treatment.
Clinical trial registration: NCT04860323.
Keywords: ATI; Africa; HIV cure; HIV remission; broadly neutralizing antibodies; stakeholder engagement.
© 2025 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Chun TW, Fauci AS. HIV reservoirs. AIDS. 2012;26(10):1261–8. - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV‐1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–300. - PubMed
-
- Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication‐competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

