Elevation of ISG15 promotes diabetic kidney disease by modulating renal tubular epithelial cell pyroptosis
- PMID: 40462493
- PMCID: PMC12134392
- DOI: 10.1002/ctm2.70337
Elevation of ISG15 promotes diabetic kidney disease by modulating renal tubular epithelial cell pyroptosis
Abstract
Background: Fibrosis and inflammation in the renal tubular epithelial cells (TECs) are key contributors to the pathology of diabetic kidney disease (DKD). Nevertheless, the precise triggers of these processes remain unclear. This study aimed to explore the role of interferon-stimulated gene 15 (ISG15) in the injury of TECs induced by high glucose (HG) conditions and its implications for the development of DKD.
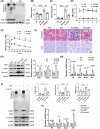
Methods: ISG15 knockout (ISG15 KO) mice injected with streptozotocin-treated mice on a high-fat diet were used to investigate its role in DKD. Cellular models with ISG15 knockdown were exposed to HG conditions to assess the effects of ISG15 on cellular responses. Subsequently, we evaluated the impact of ISG15 on pyroptosis, a form of programmed cell death, to understand its potential role in DKD pathology. Furthermore, RNA sequencing (RNA-seq) and molecular biology techniques were employed to explore the signalling pathways potentially regulated by ISG15.
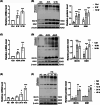
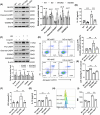
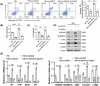
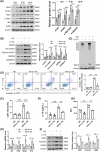
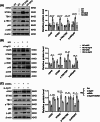
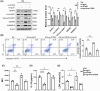
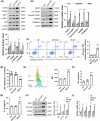
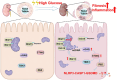
Results: We first confirmed an up-regulation of ISG15 within the renal tubule in DKD. The deletion of ISG15 alleviated renal functional damage, fibrosis and inflammation, which correlated with reduced ISGylation levels. Mechanistic investigation revealed that HG stimulation in TECs disrupted the mtDNA-cGAS-STING signalling, which exacerbates the DKD through the NLRP3-CASP1-GSDMD axis. Furthermore, we uncovered a bidirectional regulatory loop between STING and ISG15, with STING enhancing ISG15 expression upstream and ISG15 modulating STING expression through ISGylation.
Conclusion: ISG15-mtDNA-STING emerges as a critical hub that integrates the processes of pyroptosis, fibrosis and inflammation. Therapeutic interventions that target this signalling network at various levels may pave the way for innovative treatments for DKD.
Key points: ISG15 is highly expressed in both DKD mice and renal tubular epithelial cell cultured in HG condition. ISG15 promotes DKD pyroptosis via NLRP3-CASP1-GSDMD axis. ISG15-mtDNA-STING emerges as a critical hub that integrates the processes of pyroptosis.
Keywords: ISG15; diabetic kidney disease; fibrosis; pyroptosis; renal tubular epithelial cells.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The axis of miR-30a/AIF-1/TRPC6/calcineurin A/NFAT2 regulated the death modalities and inflammation of renal tubular epithelial cells in diabetic kidney disease via exosome.Life Sci. 2025 Sep 15;377:123760. doi: 10.1016/j.lfs.2025.123760. Epub 2025 May 24. Life Sci. 2025. PMID: 40419106
-
PDIA4 targets IRE1α/sXBP1 to alleviate NLRP3 inflammasome activation and renal tubular injury in diabetic kidney disease.Biochim Biophys Acta Mol Basis Dis. 2025 Mar;1871(3):167645. doi: 10.1016/j.bbadis.2024.167645. Epub 2024 Dec 30. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39743023
-
(Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway.J Transl Med. 2024 Jan 5;22(1):26. doi: 10.1186/s12967-023-04846-5. J Transl Med. 2024. PMID: 38183100 Free PMC article.
-
New Insights into the Mechanisms of Pyroptosis and Implications for Diabetic Kidney Disease.Int J Mol Sci. 2020 Sep 25;21(19):7057. doi: 10.3390/ijms21197057. Int J Mol Sci. 2020. PMID: 32992874 Free PMC article. Review.
-
Relevance of the Pyroptosis-Related Inflammasome Pathway in the Pathogenesis of Diabetic Kidney Disease.Front Immunol. 2021 Feb 22;12:603416. doi: 10.3389/fimmu.2021.603416. eCollection 2021. Front Immunol. 2021. PMID: 33692782 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
