Comparative Analysis of SiMoA and Ella Immunoassay Platforms for Measuring Serum Neurofilament Light Chain Levels in ATTRv With Polyneuropathy and Presymptomatic Carriers
- PMID: 40462552
- PMCID: PMC12134389
- DOI: 10.1111/ene.70215
Comparative Analysis of SiMoA and Ella Immunoassay Platforms for Measuring Serum Neurofilament Light Chain Levels in ATTRv With Polyneuropathy and Presymptomatic Carriers
Abstract
Background: Neurofilament light chains (NfL) represent reliable serum biomarkers of neuroaxonal injury. Due to their low serum levels, precise detection methods are critical. This study aimed to scrutinize the comparability of two techniques: Single Molecule Array (SiMoA) and Ella automated immunoassay, analyzing serum NfL levels in ATTRv presymptomatic subjects and polyneuropathy patients.
Methods: Blood samples were processed and analyzed using commercial Ella and SiMoA kits. Statistical analysis included the Wilcoxon signed-rank test, Spearman correlation, Bland-Altman, and Passing-Bablok regression. ANCOVA models were used to compare NfL levels between cohorts.
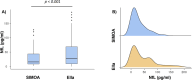
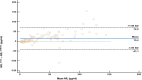
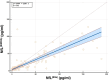
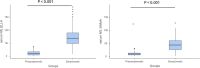
Results: The study measured serum NfL levels in 55 symptomatic and 55 presymptomatic ATTRv subjects. Median NfL concentrations were significantly higher with Ella (median 27.5 pg/mL) than SiMoA (median 15.9 pg/mL). Both methods showed a strong positive correlation (r = 0.8, p < 0.001), but Ella overestimated NfL by 42%. Bland-Altman analysis revealed a mean bias of 15.4 pg/mL, with limits of agreement between -41.1 and 72.0 pg/mL. The slope of the Passing-Bablok regression line was 0.58, and the intercept was 3.48 pg/mL, suggesting that the Ella platform tends to overestimate NfL concentrations compared to the SiMoA platform, especially at higher concentrations. Both methods effectively distinguished presymptomatic from symptomatic patients (p < 0.001 for both).
Conclusions: Our findings underscore that both platforms are effective in measuring serum NfL, with Ella consistently overestimating, especially at higher concentrations. The difference between the two platforms must be taken into account when deeming the concentrations as pathological or normal, as well as when conducting longitudinal studies.
Keywords: SiMoA; biomarker; ella; method comparison; neurofilament light chain.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Khalil M., Teunissen C. E., Lehmann S., et al., “Neurofilaments as Biomarkers in Neurological Disorders ‐ Towards Clinical Application,” Nature Reviews. Neurology 20 (2024): 269–287. - PubMed
-
- Bellanti R., Keddie S., Lunn M. P., and Rinaldi S., “Ultrasensitive Assay Technology and Fluid Biomarkers for the Evaluation of Peripheral Nerve Disease,” Journal of Neurology, Neurosurgery & Psychiatry 95, no. 2 (2023): 114–124. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

