P23H rhodopsin accumulation causes transient disruptions to synaptic protein levels in rod photoreceptors in a model of retinitis pigmentosa
- PMID: 40462724
- PMCID: PMC12233068
- DOI: 10.1242/dmm.052256
P23H rhodopsin accumulation causes transient disruptions to synaptic protein levels in rod photoreceptors in a model of retinitis pigmentosa
Abstract
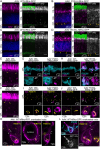
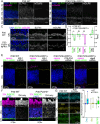
Rod photoreceptor neurons in the retina detect scotopic light through the visual pigment rhodopsin (Rho) in their outer segment (OS). Efficient Rho trafficking to the OS through the inner rod compartments is critical for long-term rod health. However, given the importance of protein trafficking to the OS, little is known about the trafficking of rod synaptic proteins. Furthermore, the subcellular impact of Rho mislocalization on rod synapses (i.e. 'spherules') has not been investigated. In this study, we used super-resolution and electron microscopies, along with proteomics, to perform a subcellular analysis of Rho synaptic mislocalization in P23H-Rho-RFP mutant mice. We discovered that mutant P23H-Rho-RFP protein mislocalized in distinct accumulations within the spherule cytoplasm, which we confirmed with adeno-associated virus overexpression. Additionally, we found specific synaptic protein abundance differences in P23H-Rho-RFP mice. Interestingly, in P23H knock-in mice with no RFP tag, we detected no synaptic protein abundance changes. In rd10 mutant rods, Rho mislocalized along the spherule plasma membrane, and there were synaptic protein abundance differences at postnatal day 20. Our findings demonstrate that some rod photoreceptor synaptic proteins are sensitive to Rho mislocalization.
Keywords: Mislocalization; Photoreceptors; Retina; Rhodopsin; Super-resolution; Synapse.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures






Update of
-
P23H rhodopsin aggregation in the ER causes synaptic protein imbalance in rod photoreceptors.bioRxiv [Preprint]. 2024 Dec 16:2024.10.18.619115. doi: 10.1101/2024.10.18.619115. bioRxiv. 2024. Update in: Dis Model Mech. 2025 Jun 1;18(6):dmm052256. doi: 10.1242/dmm.052256. PMID: 39484588 Free PMC article. Updated. Preprint.
References
-
- Adly, M. A., Spiwoks-Becker, I. and Vollrath, L. (1999). Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Invest. Ophthalmol. Vis. Sci. 40, 2165-2172. - PubMed
-
- Agrawal, S. A., Burgoyne, T., Eblimit, A., Bellingham, J., Parfitt, D. A., Lane, A., Nichols, R., Asomugha, C., Hayes, M. J., Munro, P. M.et al. (2017). REEP6 deficiency leads to retinal degeneration through disruption of ER homeostasis and protein trafficking. Hum. Mol. Genet. 26, 2667-2677. 10.1093/hmg/ddx149 - DOI - PMC - PubMed
-
- Athanasiou, D., Aguila, M., Bellingham, J., Li, W., McCulley, C., Reeves, P. J. and Cheetham, M. E. (2018). The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 62, 1-23. 10.1016/j.preteyeres.2017.10.002 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

