Intra-amniotic infection with Ureaplasma parvum causes serovar-dependent white matter damage in preterm fetal sheep
- PMID: 40462859
- PMCID: PMC12130620
- DOI: 10.1093/braincomms/fcaf182
Intra-amniotic infection with Ureaplasma parvum causes serovar-dependent white matter damage in preterm fetal sheep
Abstract
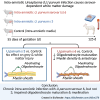
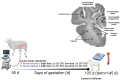
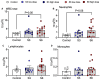
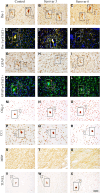
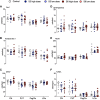
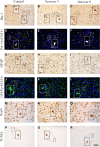
Ureaplasma parvum is commonly isolated from the amniotic fluid of pregnancies complicated by infection. While some studies have shown an association between intra-amniotic Ureaplasma species infection and brain injury and/or adverse neurodevelopment, others have not. The relationship between antenatal exposure to microbial infection and risk of poor neurological outcome is complex and multifactorial and may reflect diversities in microbial pathogenicity along with the duration and severity of the fetal inflammatory response to microbial infection. This study aimed to determine the impact of chronic intra-amniotic infection with Ureaplasma parvum serovars 3 and 6, which are among the most common serovars isolated in pregnancies complicated by infection/inflammation, on white matter development in preterm fetal sheep. Pregnant ewes carrying singleton or twin fetuses (55 days gestational age, term = 145 days) were randomly allocated to receive an ultrasound-guided intra-amniotic injection of Ureaplasma parvum serovars 3 (n = 11), 6 (n = 16) or media (control, n = 6). At 125 days of gestation, the ewe and foetus were euthanized and the fetal brain was collected for immunohistochemistry. Total numbers of oligodendrocytes (oligodendrocyte transcription factor 2-positive cells) in the periventricular white matter tract were higher in Ureaplasma parvum serovar 6-exposed fetuses than control. Numbers of mature oligodendrocytes [anti-adenomatous polyposis coli clone (CC) 1-positive cells] and myelin density (% area fraction of myelin basic protein-positive) in the periventricular and intragyral white matter tracts were lower in Ureaplasma parvum serovar 6-exposed fetuses than control. Myelin anisotropy was lower in serovar 6-exposed fetuses than control. There were no differences in numbers of total or mature oligodendrocytes, myelin density and anisotropy in Ureaplasma parvum serovar-3-exposed fetuses compared to control. Cell death, numbers of neurons, total and reactive (signal transducer and activator of transcription 3-positive) microglia and astrocytes did not differ between Ureaplasma parvum-exposed fetuses and controls within the premotor cortex and striatum. Chronic intra-amniotic infection with Ureaplasma parvum serovar 6, but not 3, impaired oligodendrocyte maturation and myelination within the large white matter tracts of the preterm sheep brain. These data suggest that the impact Ureaplasma parvum infection on white matter development may be serovar dependant, which may help to explain why some fetuses exposed to intra-amniotic Ureaplasma infection have adverse neurodevelopmental outcomes while others do not. Overall, this study demonstrates that greater emphasis needs to be placed on the taxonomy of Ureaplasma infection when designing and interpreting clinical and preclinical studies of fetal infection and neurodevelopmental outcomes.
Keywords: Ureaplasma; chorioamnionitis; infection; preterm brain injury; white matter injury.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Kikhney J, von Schöning D, Steding I, et al. Is Ureaplasma spp. the leading causative agent of acute chorioamnionitis in women with preterm birth? Clin Microbiol Infect. 2017;23(2):119.e1–119.e7. - PubMed
-
- Leli C, Mencacci A, Latino MA, et al. Prevalence of cervical colonization by Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in childbearing age women by a commercially available multiplex real-time PCR: An Italian observational multicentre study. J Microbiol Immunol Infect. 2018;51(2):220–225. - PubMed
LinkOut - more resources
Full Text Sources
