Radioembolization for neuroendocrine tumors: procedure, application and clinical outcomes
- PMID: 40462865
- PMCID: PMC12131736
- DOI: 10.1530/EO-24-0053
Radioembolization for neuroendocrine tumors: procedure, application and clinical outcomes
Abstract
Neuroendocrine liver metastases significantly affect patient prognosis and quality of life due to their symptomatic burden and challenging management. Besides conventional systemic therapies, liver-directed therapies improve patient outcomes in patients with liver-dominant disease. These liver-directed therapies have gained interest over the past decade, but their placement in the treatment algorithm of neuroendocrine liver metastases remains largely unclear. The purpose of this review is to evaluate the current role of selective internal radiation therapy (radioembolization) as a treatment for neuroendocrine liver metastases. This review examines the patient selection, procedural aspects, applications, and clinical outcomes. Radioembolization is effective as a standalone treatment. This treatment achieves disease control rates exceeding 90% and improves symptoms and quality of life. Moreover, combining radioembolization with systemic therapies may provide improved treatment response and additional benefits, but further investigation is required. The treatments effectiveness is influenced by appropriate patient selection, including consideration of liver function, tumor vascularity and previous interventions. A multidisciplinary approach is essential in assessing treatment eligibility. Patient management should be tailored on an individual level to optimize outcomes. The incidence of complications is rare (<1%), with radiation-induced liver disease being the most concerning. This review underscores the need for continued research to better understand the optimal use of radioembolization. Specifically, its placement within treatment, particularly in combination with other therapies, requires further exploration, ultimately to improve survival and quality of life for patients with neuroendocrine liver metastases.
Keywords: NET; SIRT; neuroendocrine neoplasm; radioembolization.
© the author(s).
Conflict of interest statement
LSH is supported by a research grant from NWO (NWO P20-57). TB has acted as consultant for Boston Scientific and Sirtex Medical. MGEHL has acted as consultant for Boston Scientific and Terumo. AJATB has acted as consultant for Boston Scientific and Terumo.
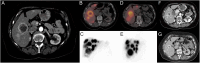
Figures

References
-
- Braat AJAT, Kwekkeboom DJ, Kam BLR, et al. 2018. Additional hepatic 166Ho-radioembolization in patients with neuroendocrine tumours treated with 177Lu-DOTATATE; a single center, interventional, non-randomized, non-comparative, open label, phase II study (HEPAR PLUS trial). BMC Gastroenterol 18 84. ( 10.1186/s12876-018-0817-8) - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
