This is a preprint.
Recombinant protein platform for high-throughput investigation of peptide-liposome interactions via fluorescence anisotropy depolarization
- PMID: 40463003
- PMCID: PMC12132265
- DOI: 10.1101/2025.05.12.653516
Recombinant protein platform for high-throughput investigation of peptide-liposome interactions via fluorescence anisotropy depolarization
Abstract
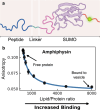
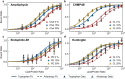
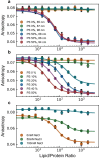
Many cytosolic proteins critical to membrane trafficking and function contain an unstructured domain that can bind to specific membranes, with a transition into an amphipathic helix induced upon membrane association. These inducible amphipathic helices often play a critical role in organelle recognition and subsequent function by these cytosolic proteins, but the tools and techniques used to characterize affinity towards specific membranes are low-throughput and highly dependent on the solubility of the inducible amphipathic helix. Here, we introduce a modular recombinant protein platform for rapidly measuring the binding affinity of inducible amphipathic helices towards a variety of membrane compositions and curvatures using high-throughput fluorescence anisotropy measurements. Inducible amphipathic helices are solubilized with a fluorescently tagged small ubiquitin-like modifier (SUMO) protein and binding to membranes quantified by leveraging the unexpected decrease in fluorescence anisotropy upon binding, a phenomenon previously observed but not well understood. By using fluorescence anisotropy decay measurements and solution NMR experiments, we deduce that this phenomenon likely occurs due to the local increase in fluorophore motion upon binding to the membrane. Altogether, this recombinant protein platform can be readily applied to any inducible amphipathic helix of interest, allowing for detailed investigation of the specific membrane biochemical parameters facilitating binding.
Keywords: Amphipathic helix; fluorescence anisotropy depolarization; high-throughput protein vesicle binding; peptide-liposome interactions; vesicles.
Conflict of interest statement
Competing Interest Statement: The authors declare no competing interest.
Figures


Similar articles
-
Site-specific tryptophan dynamics in class A amphipathic helical peptides at a phospholipid bilayer interface.Biophys J. 2000 Aug;79(2):1066-73. doi: 10.1016/S0006-3495(00)76360-0. Biophys J. 2000. PMID: 10920036 Free PMC article.
-
An Amphipathic Alpha-Helix Domain from Poliovirus 2C Protein Tubulate Lipid Vesicles.Viruses. 2020 Dec 18;12(12):1466. doi: 10.3390/v12121466. Viruses. 2020. PMID: 33353144 Free PMC article.
-
Evidence for the amphipathic nature and tilted topology of helices 4 and 5 in the closed state of the colicin E1 channel.Biochemistry. 2009 Feb 17;48(6):1369-80. doi: 10.1021/bi801906v. Biochemistry. 2009. PMID: 19159330
-
BAR domains, amphipathic helices and membrane-anchored proteins use the same mechanism to sense membrane curvature.FEBS Lett. 2010 May 3;584(9):1848-55. doi: 10.1016/j.febslet.2010.01.053. Epub 2010 Jan 31. FEBS Lett. 2010. PMID: 20122931 Review.
-
Recognizing asymmetry in pseudo-symmetry; structural insights into the interaction between amphipathic α-helices and X-bundle proteins.Biochim Biophys Acta Proteins Proteom. 2017 Nov;1865(11 Pt B):1605-1612. doi: 10.1016/j.bbapap.2017.06.017. Epub 2017 Jun 23. Biochim Biophys Acta Proteins Proteom. 2017. PMID: 28652208 Review.
References
-
- Bussell R. & Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J. Mol. Biol. 329, 763–778 (2003). - PubMed
-
- Cornell R. B. Membrane lipid compositional sensing by the inducible amphipathic helix of CCT. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1861, 847–861 (2016). - PubMed
-
- Puth K., Hofbauer H. F., Sáenz J. P. & Ernst R. Homeostatic control of biological membranes by dedicated lipid and membrane packing sensors. Biol. Chem. 396, 1043–1058 (2015). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
