This is a preprint.
Liver-Derived, Circulating Xanthine Oxidoreductase Drives Vascular Impairment Associated with Inhalation of Ultrafine Particulates
- PMID: 40463028
- PMCID: PMC12132582
- DOI: 10.1101/2025.05.07.652261
Liver-Derived, Circulating Xanthine Oxidoreductase Drives Vascular Impairment Associated with Inhalation of Ultrafine Particulates
Abstract
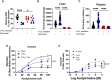
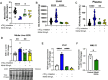
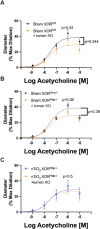
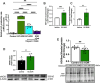
Inhalation of ultrafine particles (UFP) mediates systemic vascular impairment which is, in part, driven by elevated rates of oxidant generation. One significant source of oxidant production in the vascular compartment is the purine catabolizing enzyme, xanthine oxidoreductase (XOR). However, mechanisms linking XOR and/or endothelial glycosaminoglycan (GAG)-sequestered XOR to vessel dysfunction allied to UFP inhalation remain underexplored. Based on known interactions between UFP and the liver, we hypothesized that exposure could lead to hepatic release of XOR to the circulation which subsequently contributes to vascular impairment. Utilizing our murine hepatocyte-specific XOR knockout (XORHep-/-) model (loss of function) in conjunction with reintroducing exogenous XOR (restoration of function) we demonstrate a specific role for liver-derived XOR in the pathogenesis of UFP-induced vascular impairment. Exposure of mice as well as in vitro exposure of hepatocytes to our model UFP, nano titanium dioxide (nTiO2) results in the upregulation and active release of XOR. Drinking water supplemented with the XOR inhibitor febuxostat or nitrite ( ) partially prevented nTiO2-induced impairment of vascular reactivity. Interestingly, nitrite appears to cause a down-regulation of hepatic XOR. XORHep-/- mice were partially protected against both impairment of endothelial dependent dilation and augmented angiotensin II constriction. To further demonstrate the role of circulating XOR in nTiO2-induced impairment of vessel reactivity, XORHep-/- mice had circulating XOR restored by i.v. injection prior to exposure, which eliminated the protection of the hepatic knockout. It is important to note that acute restoration of intraluminal XOR in isolated vessels did not alter endothelial-dependent dilation or angiotensin II constriction. As such, we interrogated potential downstream mediators of XOR effects on endothelial function and found a decrease in the repressive trimethylation of lysine 9 on histone 3. Together these findings demonstrate that circulating XOR is a key contributor to endothelial dysfunction caused by UFP exposure. However, the impairment is not acute in nature and might involve epigenetic-mediated alterations in gene expression.
Keywords: glycosaminoglycans; nano-TiO2; nitrate; nitric oxide; nitrite; oxidants; xanthine oxidase.
Figures





References
-
- Saha PK, Hankey S, Marshall JD, Robinson AL, Presto AA. High-Spatial-Resolution Estimates of Ultrafine Particle Concentrations across the Continental United States. Environ Sci Technol. 2021;55(15):10320–10331. - PubMed
-
- Khraishah H, Alahmad B, Ostergard RL Jr., et al. Climate change and cardiovascular disease: implications for global health. Nat Rev Cardiol. 2022. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
