This is a preprint.
The distribution of fitness effects varies phylogenetically across animals
- PMID: 40463062
- PMCID: PMC12132501
- DOI: 10.1101/2025.05.13.653358
The distribution of fitness effects varies phylogenetically across animals
Abstract
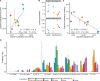
The distribution of fitness effects (DFE) describes the selection coefficients ( ) of newly arising mutations and fundamentally influences population genetic processes. However, the extent and mechanisms of DFE variation have not been systematically investigated across species with divergent phylogenetic histories and ecological functions. Here, we inferred the DFE in natural populations of eleven animal (sub)species, including humans, mice, fin whales, vaquitas, wolves, collared flycatchers, pied flycatchers, halictid bees, Drosophila, and mosquitoes. We find that the DFE co-varies with phylogeny, where the expected mutation effects are more similar in closely related species ( ). Additionally, mammals have a higher proportion of strongly deleterious mutations (22% to 47% in mammals; 0.0% to 5.4% in insects and birds) and a lower proportion of weakly deleterious mutations than insects and birds. Population size is significantly negatively correlated with the expected impact of new deleterious mutations ( ), and the proportion of new beneficial mutations ( ). These findings align with Fisher's Geometric Model (FGM), which defines organismal complexity as the number of phenotypes under selection. Consistent with the FGM's predictions, we observe that mutations are more deleterious in complex organisms, while beneficial mutations occur more frequently in smaller populations to compensate for the drift load. Our study demonstrates strong phylogenetic constraints in the evolution of a fundamental population genetics parameter, and proposes that, through mechanisms of global epistasis, long-term population size and organismal complexity drive variation in the DFE across animals.
Keywords: Fisher’s geometric model; deleterious variations; distribution of selection coefficients; effective population size; organismal complexity.
Figures





Similar articles
-
Determining the factors driving selective effects of new nonsynonymous mutations.Proc Natl Acad Sci U S A. 2017 Apr 25;114(17):4465-4470. doi: 10.1073/pnas.1619508114. Epub 2017 Apr 11. Proc Natl Acad Sci U S A. 2017. PMID: 28400513 Free PMC article.
-
Comparison of the Full Distribution of Fitness Effects of New Amino Acid Mutations Across Great Apes.Genetics. 2019 Nov;213(3):953-966. doi: 10.1534/genetics.119.302494. Epub 2019 Sep 5. Genetics. 2019. PMID: 31488516 Free PMC article.
-
The evolutionarily stable distribution of fitness effects.Genetics. 2015 May;200(1):321-9. doi: 10.1534/genetics.114.173815. Epub 2015 Mar 10. Genetics. 2015. PMID: 25762525 Free PMC article.
-
A general multivariate extension of Fisher's geometrical model and the distribution of mutation fitness effects across species.Evolution. 2006 May;60(5):893-907. Evolution. 2006. PMID: 16817531 Review.
-
Effects of new mutations on fitness: insights from models and data.Ann N Y Acad Sci. 2014 Jul;1320(1):76-92. doi: 10.1111/nyas.12460. Epub 2014 May 30. Ann N Y Acad Sci. 2014. PMID: 24891070 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
