This is a preprint.
Unreliable homeostatic action potential broadening in cultured dissociated neurons
- PMID: 40463064
- PMCID: PMC12132517
- DOI: 10.1101/2025.05.09.653135
Unreliable homeostatic action potential broadening in cultured dissociated neurons
Abstract
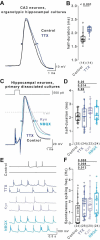
Homeostatic plasticity preserves neuronal activity against perturbations. Recently, somatic action potential broadening was proposed as a key homeostatic adaptation to chronic inactivity in neocortical neurons. Since action potential shape critically controls calcium entry and neuronal function, broadening provides an attractive homeostatic feedback mechanism to regulate activity. Here, we report that chronic inactivity induced by sodium channel block does not broaden action potentials in neocortical neurons under a wide range of conditions. In contrast, action potentials were broadened in CA3 neurons of organotypic hippocampal cultures by chronic sodium channel block and in hippocampal dissociated cultures by chronic synaptic block. Mechanistically, BK-type potassium channels were proposed to underly inactivity-induced action potential broadening. However, BK channels did not affect action potential duration in our recordings. Our results indicate that action potential broadening can occur in specific neurons and conditions but is not a general mechanism of homeostatic plasticity in cultured neurons.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Borst J.G.G., and Sakmann B. (1999). Effect of changes in action potential shape on calcium currents and transmitter release in a calyx-type synapse of the rat auditory brainstem. Philosophical Transactions of the Royal Society B: Biological Sciences 354, 347–355. 10.1098/RSTB.1999.0386. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
