This is a preprint.
A functionally validated TCR-pMHC database for TCR specificity model development
- PMID: 40463065
- PMCID: PMC12132471
- DOI: 10.1101/2025.04.28.651095
A functionally validated TCR-pMHC database for TCR specificity model development
Abstract
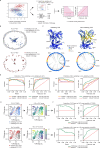
Accurate prediction of TCR specificity forms a holy grail in immunology and large language models and computational structure predictions provide a path to achieve this. Importantly, current TCR-pMHC prediction models have been trained and evaluated using historical data of unknown quality. Here, we develop and utilize a high-throughput synthetic platform for TCR assembly and evaluation to assess a large fraction of VDJdb-deposited TCR-pMHC entries using a standardized readout of TCR function. Strikingly, this analysis demonstrates that claimed TCR reactivity is only confirmed for 50% of evaluated entries. Intriguingly, the use of TCRbridge to analyze AlphaFold3 confidence metrics reveals a substantial performance in distinguishing functionally validating and non-validating TCRs even though AlphaFold3 was not trained on this task, demonstrating the utility of the validated VDJdb (TCRvdb) database that we generated. We provide TCRvdb as a resource to the community to support training and evaluation of improved predictive TCR specificity models.
Keywords: T cell receptor; functional genetic screening; major histocompatibility antigen; peptide; predictive models.
Figures



References
-
- Mora T. & Walczak A. M. How many different clonotypes do immune repertoires contain? Curr. Opin. Syst. Biol. 18, 104–110 (2019).
-
- Schumacher T. N., Scheper W. & Kvistborg P. Cancer Neoantigens. Annu. Rev. Immunol. 37, 173–200 (2019). - PubMed
-
- Zinkernagel R. M. & Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytes choriomeningitis. Nature 251, 547–548 (1974). - PubMed
-
- Hedrick S. M., Cohen D. I., Nielsen E. A. & Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature 308, 149–153 (1984). - PubMed
-
- Yanagi Y. et al. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature 308, 145–149 (1984). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
