This is a preprint.
Regulation of the Yolk Microtubule and Actin Cytoskeleton by Dachsous Cadherins during Zebrafish Epiboly
- PMID: 40463075
- PMCID: PMC12132318
- DOI: 10.1101/2025.05.10.653271
Regulation of the Yolk Microtubule and Actin Cytoskeleton by Dachsous Cadherins during Zebrafish Epiboly
Abstract
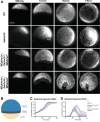
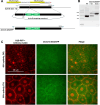
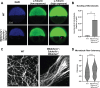
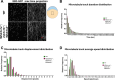
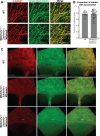
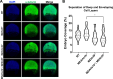
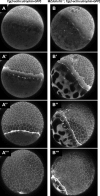
Epiboly is a crucial morphogenetic process during early animal embryogenesis that expands surface area of embryonic tissues while thinning them. During zebrafish development, epiboly spreads the superficial enveloping layer (EVL), germ layers, and yolk syncytial layer to cover the yolk cell. Here we investigated functions of the three zebrafish dchs genes, dchs1a, dchs1b and dchs2 that encode large atypical cadherins and report that they have partially overlapping functions in epiboly progression. We have inserted GFP at the C-terminal Dchs1b intracellular domain of the endogenous dchs1b locus using homologous recombination. We observed the resulting Dchs1b-GFP fusion protein localized in both the cell membrane and the cytoplasm of EVL and embryonic cells during gastrulation. The dynamic microtubule and actin cytoskeleton of the yolk cell are essential for epiboly. Our studies of the yolk microtubule network demonstrate that these microtubules are more bundled and show faster polymerization during epiboly in dchs triple loss-of-function mutant embryos than in wild-type embryos, indicating that dchs genes are required for limiting microtubule polymerization and promoting dynamics during epiboly. The epiboly progression defects of dchs1b deficient mutants were suppressed by mutations in the tetratricopeptide repeat protein 28 (ttc28) gene encoding a cytoplasmic protein previously shown to bind to Dchs1b intracellular domain and alter microtubule dynamics during early cleavages. We further demonstrate that MZdchs1b mutants exhibit abnormal organization and dynamics of yolk cell actin cytoskeleton during epiboly. Together, these lines of evidence as well as our transcriptomic analyses support the notion that like during early embryonic cleavages, Dchs1b plays a major role, while Dchs1a and Dchs2 proteins have supporting roles in regulating microtubule dynamics and organization of both microtubule and actin cytoskeleton to ensure normal epiboly.
Conflict of interest statement
Declaration of interest The authors have no financial and personal relationships with other people or organizations that could inappropriately influence their work.
Figures

References
-
- Bruce A.E.E., Heisenberg C.-P., 2020. Chapter Eleven - Mechanisms of zebrafish epiboly: A current view, in: Solnica-Krezel L. (Ed.), Current Topics in Developmental Biology. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
