This is a preprint.
When should adaptation arise from a polygenic response versus few large effect changes?
- PMID: 40463133
- PMCID: PMC12132454
- DOI: 10.1101/2025.05.15.654234
When should adaptation arise from a polygenic response versus few large effect changes?
Abstract
The question of when adaptation involves genetic changes of large effect versus a polygenic response traces back to early debates around Darwin's "Origin of Species" and remains unanswered today. While there are compelling reasons to expect polygenic adaptation to be common, direct evidence for it is still lacking. In turn, there are hundreds of examples of large effect adaptations across species, but it is unclear whether they are a common occurrence in any given species. Synthesizing the different lines of evidence is further complicated by differences in study designs, limitations and biases. Here, we reframe this long-standing question in terms of the trait under selection and ask how the genetic basis of adaptation is expected to depend on key properties of the genetic variation in the trait (i.e., the trait genetics) and on the changes in selection pressures that act on it (i.e., the "trait ecology"). To study this question, we consider a quantitative trait subject to stabilizing selection and model the response to selection when a population at mutation-selection-drift balance experiences a sudden shift in the optimal value. Using this model, we delimit how the contributions of large effect and polygenic changes to adaptation depend on the genetics and ecology of the trait, as well as other salient factors. This theory allows us to formulate testable predictions about when different modes of adaptation are expected and to outline a framework within which to interpret disparate sources of evidence about the genetic basis of adaptation.
Figures


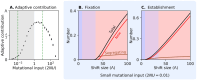

References
-
- Agrawal AA, Hastings AP, Lenhart PA, Blecher M, Duplais C, Petschenka G, et al. 2024. Convergence and Divergence among Herbivorous Insects Specialized on Toxic Plants: Revealing Syndromes among the Cardenolide Feeders across the Insect Tree of Life. The American Naturalist. 204(3):201–220. 10.1086/731277. - DOI - PubMed
-
- Andreassen OA, Thompson WK, Schork AJ, Ripke S, Mattingsdal M, Kelsoe J, et al. 2013. Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate. PLOS Genetics. 9(4):e1003455. 10.1371/journal.pgen.1003455. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
