Effect of polar organic solvents on the separation of rare earths and transition metal chloride complexes: comparison of ion exchange, extraction chromatography and solvent extraction
- PMID: 40463333
- PMCID: PMC12131135
- DOI: 10.1039/d5ra00908a
Effect of polar organic solvents on the separation of rare earths and transition metal chloride complexes: comparison of ion exchange, extraction chromatography and solvent extraction
Abstract
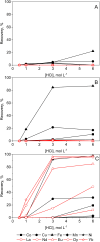
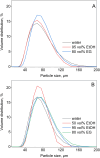
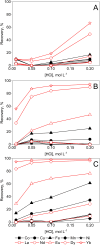
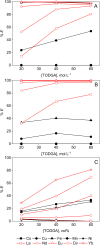
In the search for more efficient purification and separation methods for rare earths, remarkable results were obtained in the field of solvometallurgy. Replacing the aqueous phase partially or largely by polar molecular organic solvents can significantly improve extraction efficiency and selectivity in the separation of rare earths and transition metals. The effect of polar organic solvents on the sorption of rare-earth elements and transition metals was investigated for strong anion exchanger Amberlite IRA 402 (Cl-), and for extraction chromatography resins, TEVA (Cl-) and DGA. The sorption of metal ions was tested from ethanol, ethylene glycol, water and formamide. While Amberlite IRA 402 selectively recovered the iron, copper and cobalt, while leaving the rare-earth elements in solution, the DGA resin was selective for rare-earth elements and could be used for the separation of heavy rare earths from light rare earths. The efficiency of metal uptake by the resins increased with decreasing dielectric constant of the feed solvent. These results were compared to non-aqueous solvent extraction using Aliquat 336 and TODGA from the different polar organic solvents. Significant mutual miscibility observed during these solvent extraction tests demonstrated the advantage of using ion-exchange resins over solvent extraction. However, in the case of chromatographic resins, the functional molecules are only physically bonded by the resin, and loss of sorption capacity due to extractant loss was observed with both TEVA and DGA resin, especially in combination with ethanolic solutions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Xie F. Zhang T. A. Dreisinger D. Doyle F. Miner. Eng. 2014;56:10–28.
-
- Kołodyńska D., Dominika F., Gajda B., Gęga J. and Hubicki Z., in Applications of Ion Exchange – Materials in the Environment, Springer Nature, Cham, Switzerland, 2019, pp. 161–185
-
- Avdibegović D. Barbier E. Jaklič B. Škapin S. D. Spreitzer M. Binnemans K. Chemosphere. 2023;330:138603. - PubMed
-
- Moody G. J. Thomas J. D. R. Analyst. 1968;93:557–588.
-
- Hubicki Z. Olszak M. Adsorpt. Sci. Technol. 1998;16:521–528.
LinkOut - more resources
Full Text Sources

