Piezo1-Mediated Ferroptosis Delays Wound Healing in Aging Mice by Regulating the Transcriptional Activity of SLC7A11 through Activating Transcription Factor 3
- PMID: 40463502
- PMCID: PMC12133029
- DOI: 10.34133/research.0718
Piezo1-Mediated Ferroptosis Delays Wound Healing in Aging Mice by Regulating the Transcriptional Activity of SLC7A11 through Activating Transcription Factor 3
Abstract
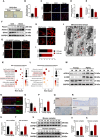
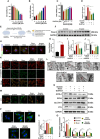
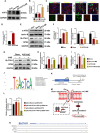
Ferroptosis plays a role in wound healing during the maturation of senescent endothelial cells. This study explores the modulation of ferroptosis in senescent human umbilical vein endothelial cells (HUVECs) and wound-healing processes by Piezo1 activation at the molecular, cellular, and tissue levels. Elevated Piezo1 expression was observed in HUVECs treated with the senescence inducer doxorubicin (Doxo) and the ferroptosis inducer erastin and in aged wound tissue. Pharmacological inhibition or knockdown of Piezo1 protected senescent HUVECs and aged wound tissue from ferroptosis. Additionally, Piezo1 channel activity was found to promote ferroptosis in senescent HUVECs by increasing intracellular Ca2+ levels. The calmodulin-dependent kinase II (CaMKII)/activating transcription factor 3 (ATF3)/SLC7A11 signaling axis was activated upon stimulation with erastin and Doxo, driving Piezo1-induced ferroptosis. CaMKII directly interacted with ATF3, which could be modulated through Piezo1 channel regulation. Notably, Piezo1 knockout mice or adeno-associated virus 9-mediated silencing of ATF3 attenuated ferroptosis in senescent cells and accelerated wound repair. Mechanistically, both genetic and pharmacological inhibition of Piezo1 promoted wound healing in aged tissues and regulated ferroptosis in senescent HUVECs through the CaMKII/ATF3/SLC7A11 pathway. In conclusion, these findings suggest that targeting Piezo1-mediated ferroptosis in senescent HUVECs offers a promising therapeutic approach for improving wound healing in the elderly.
Copyright © 2025 Chen Ji et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

