This is a preprint.
Human Applications of Transcranial Temporal Interference Stimulation: A Systematic Review
- PMID: 40463528
- PMCID: PMC12132165
- DOI: 10.1101/2025.05.16.25327804
Human Applications of Transcranial Temporal Interference Stimulation: A Systematic Review
Update in
-
Human applications of transcranial temporal interference stimulation: A systematic review.Brain Stimul. 2025 Nov-Dec;18(6):2054-2066. doi: 10.1016/j.brs.2025.10.023. Epub 2025 Oct 28. Brain Stimul. 2025. PMID: 41167554
Abstract
Background: Many neurological and psychiatric disorders involve dysregulation of subcortical structures. Transcranial temporal interference stimulation (tTIS) is a novel, non-invasive method developed to selectively modulate deep brain regions and associated neural circuits.
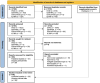
Methods: A systematic review was conducted to evaluate human applications of tTIS (PROSPERO ID: CRD42024559678). MEDLINE, Embase, APA PsycINFO, CENTRAL, ClinicalTrials.gov, and WHO ICTRP were searched up to December 12, 2024. Studies involving human applications of tTIS were eligible. Methodological quality was appraised using the NIH and modified Oxford Centre for Evidence-Based Medicine tools.
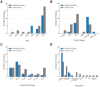
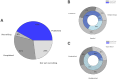
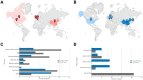
Results: Forty-eight records were reviewed (20 published studies, 28 ongoing trials). Of published studies, 16 single-session and 4 multi-session studies assessed safety, mechanistic outcomes, or therapeutic effects of tTIS in 820 participants. Stimulation was most commonly delivered at beta (20 Hz) or gamma (30-130 Hz) envelope frequencies. Neuroimaging studies support target engagement of the motor cortex, basal ganglia, and hippocampus in humans, particularly when stimulation is paired with behavioural tasks. Preliminary clinical findings in small samples demonstrated acute symptom improvements in bradykinesia and tremor within 60 minutes following a single tTIS session in Parkinson's disease and essential tremor. Reported adverse events across studies were mild (e.g., tingling, itching). Emerging trials increasingly utilize multi-session protocols (2-40 sessions) and are extending tTIS to patients with neurological and psychiatric disorders, particularly epilepsy and depression.
Conclusions: Phase 1 studies demonstrate that tTIS is safe, well-tolerated, and capable of engaging deep brain targets in humans. Well-controlled Phase 2 trials are needed to assess its therapeutic potential in patient populations.
Keywords: brain; clinical study; deep brain stimulation; electric stimulation; humans; systematic review; temporal interference.
Figures
References
-
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Psychiatry 2022;9:137–50. 10.1016/S2215-0366(21)00395-3. - DOI - PMC - PubMed
-
- Fenoy AJ, Simpson RK. Risks of common complications in deep brain stimulation surgery: management and avoidance: Clinical article. JNS 2014;120:132–9. 10.3171/2013.10.JNS131225. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
