This is a preprint.
Global multi-ancestry genetic study elucidates genes and biological pathways associated with thyroid cancer and benign thyroid diseases
- PMID: 40463558
- PMCID: PMC12132132
- DOI: 10.1101/2025.05.15.25327513
Global multi-ancestry genetic study elucidates genes and biological pathways associated with thyroid cancer and benign thyroid diseases
Abstract
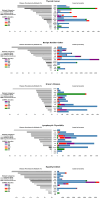
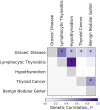
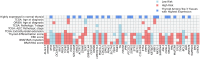
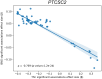
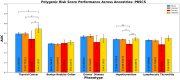
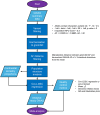
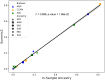
Thyroid diseases are common and highly heritable. Under the Global Biobank Meta-analysis Initiative, we performed a meta-analysis of genome-wide association studies from 19 biobanks for five thyroid diseases: thyroid cancer, benign nodular goiter, Graves' disease, lymphocytic thyroiditis, and primary hypothyroidism. We analyzed genetic association data from ~2.9 million genomes and identified 235 known and 501 novel independent variants significantly linked to thyroid diseases. We discovered genetic correlations between thyroid cancer, benign nodular goiter, and autoimmune thyroid diseases (r 2 =0.21-0.97). Telomere maintenance genes contribute to benign and malignant thyroid nodular disease risk, whereas cell cycle, DNA repair, and DNA damage response genes are predominantly associated with thyroid cancer. We proposed a paradigm explaining genetic predisposition to benign and malignant thyroid nodules. We evaluated thyroid cancer polygenic risk scores (PRS) for clinical applications in thyroid cancer diagnosis. We found PRS associations with thyroid cancer risk features: multifocality, lymph node metastases, and extranodal extension.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
