Alzheimer's disease drug development pipeline: 2025
- PMID: 40463637
- PMCID: PMC12131090
- DOI: 10.1002/trc2.70098
Alzheimer's disease drug development pipeline: 2025
Abstract
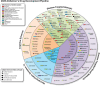
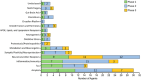
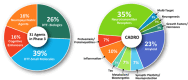
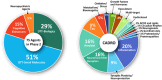
Clinical trials for Alzheimer's disease (AD) must be registered on clinicaltrials.gov. The registry presents a variety of types of information related to the planned clinical trial. We assess clinicaltrials.gov to document and compare aspects of drug development across the AD pipeline. Currently, there are 138 drugs being assessed in 182 clinical trials in the AD pipeline. Biological disease-targeted therapies (DTTs) comprise 30% of the pipeline; small molecule DTTs account for 43% of the pipeline; drugs addressing cognitive enhancement account for 14% of the pipeline; and drugs aiming to ameliorate neuropsychiatric symptoms in participants with AD contribute 11% of the pipeline. Biomarkers are among the primary outcomes of 27% of active trials. Repurposed agents represent 33% of the pipeline agents. The pipeline has more trials and more drugs compared to the 2024 pipeline.
Highlights: The 2025 Alzheimer's disease drug development pipeline hosts 182 trials and 138 novel drugs.The 2025 Alzheimer's disease drug development pipeline is diverse, with agents that address 15 basic disease processes.The 2025 Alzheimer's disease drug development pipeline has more trials and more drugs than the 2024 pipeline.Biomarkers play an important role in current trials to determine trial eligibility and as outcomes of trials.Repurposed agents comprise approximately one-third of the agents and trials in the 2025 Alzheimer's disease drug development pipeline.
Keywords: Alzheimer's disease; Common Alzheimer's Disease Research Ontology (CADRO); Phase 1; Phase 2; Phase 3; amyloid; biomarkers; clinical trials; drug development; inflammation; pharmaceutical companies; repurposed drugs; synaptic function; tau.
© 2025 The Author(s). Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
J.L.C. has provided consultation to Acadia, Acumen, ALZpath, Annovis, Aprinoia, Artery, Axsome, Biogen, Biohaven, BioXcel, Bristol‐Myers Squib, Cervomed, Eisai, Fosun, GAP Foundation, Green Valley, IGC, Janssen, Kinoxis, Lighthouse, Lilly, Lundbeck, LSP/eqt, Mangrove Therapeutics, Merck, MoCA Cognition, New Amsterdam, Novo Nordisk, NSC Therapeutics, Optoceutics, Otsuka, Oxford Brain Diagnostics, Praxis, Prothena, ReMYND, Roche, Scottish Brain Sciences, Signant Health, Simcere, sinaptica, T‐Neuro, TrueBinding, and Vaxxinity pharmaceutical, assessment, and investment companies. G.L. is a full‐time employee of Eisai Co., Ltd. K.Z. is CEO of CNS Innovations. Y.Z., J.F., A. L.‐O., and F.C. declare no competing interests. Author disclosures are available in the Supporting Information.
Figures
References
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
