Recent advances in synthetic approaches for bioactive cinnamic acid derivatives
- PMID: 40463886
- PMCID: PMC12130629
- DOI: 10.3762/bjoc.21.85
Recent advances in synthetic approaches for bioactive cinnamic acid derivatives
Abstract
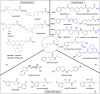
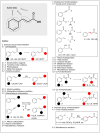
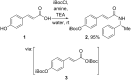
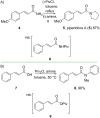
Cinnamic acid derivatives represent a significant class of biologically active compounds exhibiting a broad spectrum of activities, such as antifungal, antidengue, antimetastatic, antimicrobial, antibacterial, and anticancer properties. Their preparation has attracted considerable attention due to their versatile applications across the pharmaceutical, food, and chemical sectors. This review elucidates the functional groups of cinnamic acid that are instrumental in the rational design of biologically active derivatives. A comprehensive representative of recent advancements in synthetic methodologies over the past five years is presented, particularly emphasizing the active scaffolds of bioactive cinnamic acid derivatives. The review provides a strategic overview of alternative synthetic routes and highlights the latest innovations, including more efficient, highly selective, and environmentally sustainable approaches. Given the widespread incorporation of the cinnamic acid framework in various therapeutic agents, this review delivers critical insights into a molecular design for hit-to-lead optimization, offering detailed synthetic strategies for diverse functional modifications. By critically examining these methodologies, the paper underscores their role in expanding the utility of cinnamic acid derivatives and addressing prevailing challenges.
Keywords: biologically active; cinnamic acid derivatives; environmentally sustainable; synthetic methodologies.
Copyright © 2025, Kustiana et al.
Figures
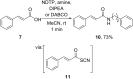
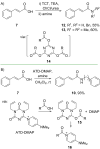
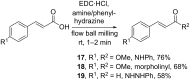
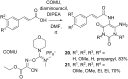
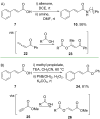
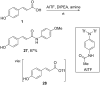
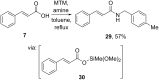
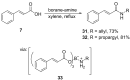
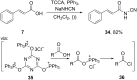
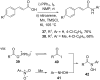
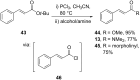
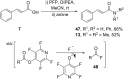
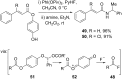
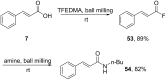
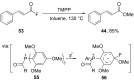
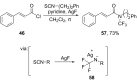
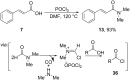
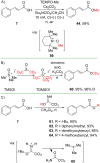
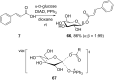
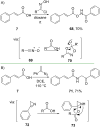
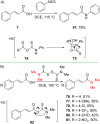
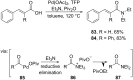
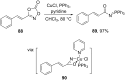
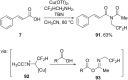
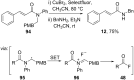

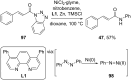
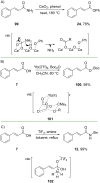
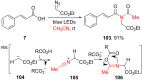
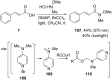
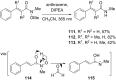
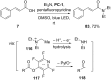
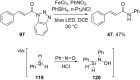
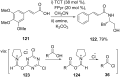
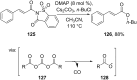
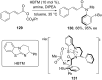
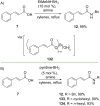
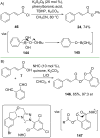
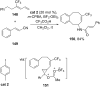
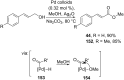
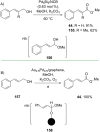
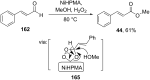
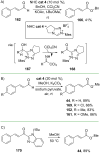
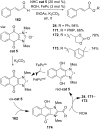
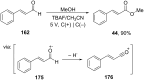
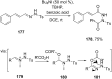
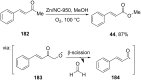
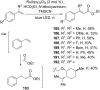
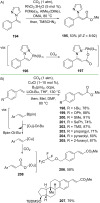
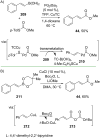
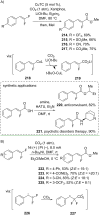
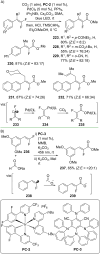
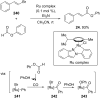
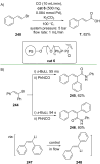
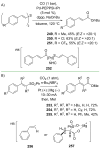
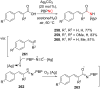
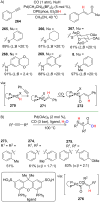
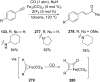
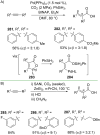
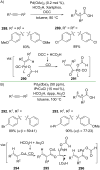
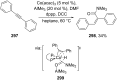
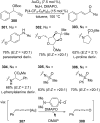
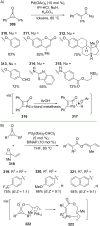
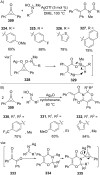
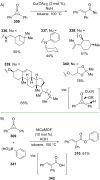
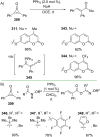
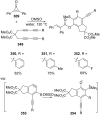
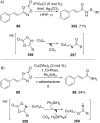
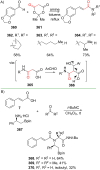
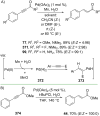
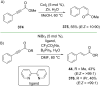
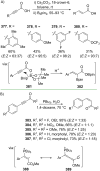

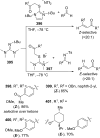
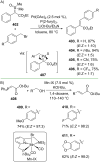
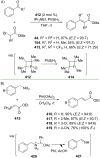
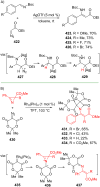
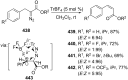
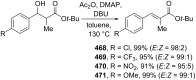
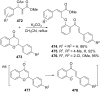
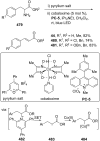
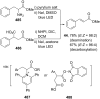
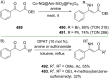
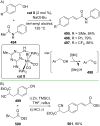
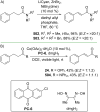
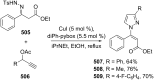
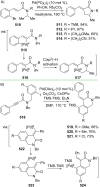
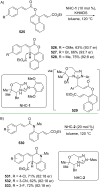
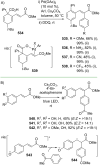
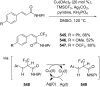
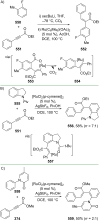
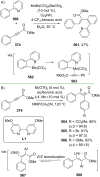
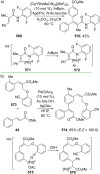
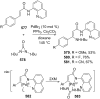
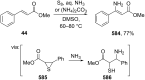
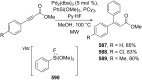
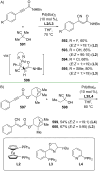
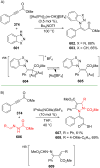
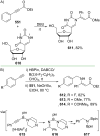
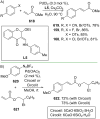
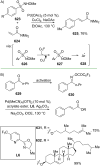
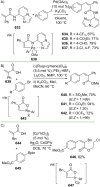
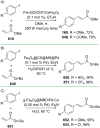
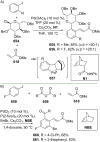
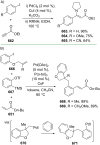
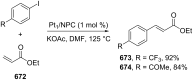
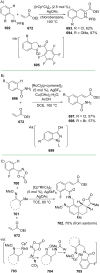
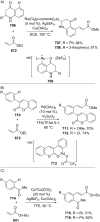
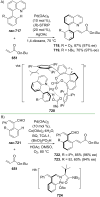
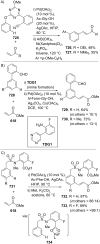
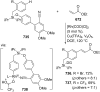
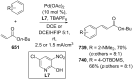
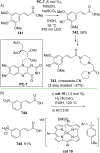
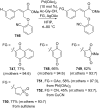
References
-
- Wiedeman P E, Djuric S W, Pilushchev M, Sciotti R J, Madar D J, Kopecka H, inventors. Oxazolidinone Chemotherapeutic Agents. US2002/0115669A1. U.S. Pat. Appl. 2002 Aug 22;
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
