Role of mitochondrial quality control in neurodegenerative disease progression
- PMID: 40463912
- PMCID: PMC12129988
- DOI: 10.3389/fncel.2025.1588645
Role of mitochondrial quality control in neurodegenerative disease progression
Abstract
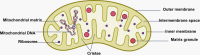
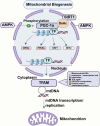
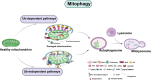
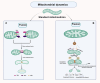
Neurodegenerative diseases are a diverse group of neurological disorders, in which abnormal mitochondrial function is closely associated with their development and progression. This has generated significant research interest in the field. The proper functioning of mitochondria relies on the dynamic regulation of the mitochondrial quality control system. Key processes such as mitochondrial biogenesis, mitophagy, and mitochondrial dynamics (division/fusion) are essential for maintaining this balance. These processes collectively govern mitochondrial function and homeostasis. Therefore, the mitochondrial quality control system plays a critical role in the onset and progression of neurodegenerative diseases. This article provides a concise overview of the molecular mechanisms involved in mitochondrial biogenesis, mitophagy, and mitochondrial dynamics, explores their interactions, and summarizes current research progress in understanding the mitochondrial quality control system in the context of neurodegenerative diseases.
Keywords: Alzheimer’s disease; Huntington’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; mitochondrial quality control.
Copyright © 2025 Liu, Sun, Guo, Yuan, Zhu, Lu, Chen, Qu, Feng and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mitochondrial dysfunction and oxidative stress in Alzheimer's disease, and Parkinson's disease, Huntington's disease and Amyotrophic Lateral Sclerosis -An updated review.Mitochondrion. 2023 Jul;71:83-92. doi: 10.1016/j.mito.2023.05.007. Epub 2023 Jun 1. Mitochondrion. 2023. PMID: 37269968 Review.
-
Role of mitophagy in mitochondrial quality control: Mechanisms and potential implications for neurodegenerative diseases.Pharmacol Res. 2021 Mar;165:105433. doi: 10.1016/j.phrs.2021.105433. Epub 2021 Jan 14. Pharmacol Res. 2021. PMID: 33454337 Review.
-
Mitophagy in Alzheimer's Disease and Other Age-Related Neurodegenerative Diseases.Cells. 2020 Jan 8;9(1):150. doi: 10.3390/cells9010150. Cells. 2020. PMID: 31936292 Free PMC article. Review.
-
Abnormal Mitochondrial Quality Control in Neurodegenerative Diseases.Front Cell Neurosci. 2020 Jun 23;14:138. doi: 10.3389/fncel.2020.00138. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32655368 Free PMC article. Review.
-
Mitophagy regulation in aging and neurodegenerative disease.Biophys Rev. 2023 Apr 4;15(2):239-255. doi: 10.1007/s12551-023-01057-6. eCollection 2023 Apr. Biophys Rev. 2023. PMID: 37124925 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

