All-Heteroatom-Substituted Carbon Spiro Stereocenters: Synthesis, Resolution, Enantiomeric Stability, and Absolute Configuration
- PMID: 40464057
- PMCID: PMC12200235
- DOI: 10.1021/jacs.5c06394
All-Heteroatom-Substituted Carbon Spiro Stereocenters: Synthesis, Resolution, Enantiomeric Stability, and Absolute Configuration
Abstract
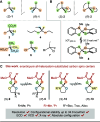
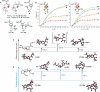
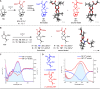
Chiral tetra-heterosubstituted methanes (i.e., tetraoxa and azatrioxa carbon spiro stereocenters) are synthesized under CpRu catalysis, using cyclic carbonates and carbamates as substrates and α-diazo-β-ketoesters as reagents. Single enantiomers, isolated by chiral stationary phase chromatography, display chiroptical properties, from gabs ∼10-5 to ∼10-4, which, together with TD-DFT calculations, provide robust absolute configuration assignments. Crystalline spiro diastereomers were also obtained, confirming further the structural and configurational assignments. Using enantioselective dynamic chromatography, remarkable enantiomerization barriers were determined for the ortho-carbonates and ortho-carbamates, with values of up to 27.6 and 34.6 kcal/mol (half-lives 227 days and >84,000 years at 25 °C, respectively). DFT further elucidates the origin of this large difference pointing toward preferred C-O or C-N bond cleavages in the rate-determining step of the SN1-like mechanism.
Figures


References
-
- Cahn R. S., Ingold C., Prelog V.. Specification of Molecular Chirality. Angew. Chem., Int. Ed. 1966;5:385–415. doi: 10.1002/anie.196603851. - DOI
- Prelog V., Helmchen G.. Basic Principles of the CIP-System and Proposals for a Revision. Angew. Chem., Int. Ed. 1982;21:567–583. doi: 10.1002/anie.198205671. - DOI
-
- Aida T., Meijer E. W., Stupp S. I.. Functional Supramolecular Polymers. Science. 2012;335:813–817. doi: 10.1126/science.1205962. - DOI - PMC - PubMed
- Zhang L., Qin L., Wang X., Cao H., Liu M.. Supramolecular Chirality in Self-Assembled Soft Materials: Regulation of Chiral Nanostructures and Chiral Functions. Adv. Mater. 2014;26:6959–6964. doi: 10.1002/adma.201305422. - DOI - PubMed
- Mabesoone M. F. J., Palmans A. R. A., Meijer E. W.. Solute–Solvent Interactions in Modern Physical Organic Chemistry: Supramolecular Polymers as a Muse. J. Am. Chem. Soc. 2020;142:19781–19798. doi: 10.1021/jacs.0c09293. - DOI - PMC - PubMed
- Mondal A., Toyoda R., Costil R., Feringa B. L.. Chemically Driven Rotatory Molecular Machines. Angew. Chem., Int. Ed. 2022;61:e202206631. doi: 10.1002/anie.202206631. - DOI - PMC - PubMed
- Pal T., Chaudhuri D.. Chiral and Morphological Anisotropy of Supramolecular Polymers Shaped by a Singularity in Solvent Composition. J. Am. Chem. Soc. 2023;145:2532–2543. doi: 10.1021/jacs.2c12253. - DOI - PubMed
- Sheng J., Pooler D. R. S., Feringa B. L.. Enlightening dynamic functions in molecular systems by intrinsically chiral light-driven molecular motors. Chem. Soc. Rev. 2023;52:5875–5891. doi: 10.1039/D3CS00247K. - DOI - PMC - PubMed
- Fu K., Zhao Y., Liu G.. Pathway-directed recyclable chirality inversion of coordinated supramolecular polymers. Nat. Commun. 2024;15:9571. doi: 10.1038/s41467-024-53928-5. - DOI - PMC - PubMed
- Chae K., Mohamad N. A. R. C., Kim J., Won D.-I., Lin Z., Kim J., Kim D. H.. The promise of chiral electrocatalysis for efficient and sustainable energy conversion and storage: a comprehensive review of the CISS effect and future directions. Chem. Soc. Rev. 2024;53:9029–9058. doi: 10.1039/D3CS00316G. - DOI - PubMed
-
- Senkuttuvan N., Komarasamy B., Krishnamoorthy R., Sarkar S., Dhanasekaran S., Anaikutti P.. The significance of chirality in contemporary drug discovery-a mini review. RSC Adv. 2024;14:33429–33448. doi: 10.1039/D4RA05694A. - DOI - PMC - PubMed
- McVicker R. U., O′Boyle N. M.. Chirality of New Drug Approvals (2013–2022): Trends and Perspectives. J. Med. Chem. 2024;67:2305–2320. doi: 10.1021/acs.jmedchem.3c02239. - DOI - PMC - PubMed
- Bloom B. P., Paltiel Y., Naaman R., Waldeck D. H.. Chiral Induced Spin Selectivity. Chem. Rev. 2024;124:1950–1991. doi: 10.1021/acs.chemrev.3c00661. - DOI - PMC - PubMed
- Adamala K. P., Agashe D., Belkaid Y., Bittencourt D. M. d. C., Cai Y., Chang M. W., Chen I. A., Church G. M., Cooper V. S., Davis M. M., Devaraj N. K., Endy D., Esvelt K. M., Glass J. I., Hand T. W., Inglesby T. V., Isaacs F. J., James W. G., Jones J. D. G., Kay M. S., Lenski R. E., Liu C., Medzhitov R., Nicotra M. L., Oehm S. B., Pannu J., Relman D. A., Schwille P., Smith J. A., Suga H., Szostak J. W., Talbot N. J., Tiedje J. M., Venter J. C., Winter G., Zhang W., Zhu X., Zuber M. T.. Confronting risks of mirror life. Science. 2024;386:1351–1353. doi: 10.1126/science.ads9158. - DOI - PubMed
- Chowdhury R., Preuss M. D., Cho H.-H., Thompson J. J. P., Sen S., Baikie T. K., Ghosh P., Boeije Y., Chua X. W., Chang K.-W., Guo E., van der Tol J., van den Bersselaar B. W. L., Taddeucci A., Daub N., Dekker D. M., Keene S. T., Vantomme G., Ehrler B., Meskers S. C. J., Rao A., Monserrat B., Meijer E. W., Friend R. H.. Circularly polarized electroluminescence from chiral supramolecular semiconductor thin films. Science. 2025;387:1175–1181. doi: 10.1126/science.adt3011. - DOI - PubMed
-
- Mislow K., Siegel J.. Stereoisomerism and local chirality. J. Am. Chem. Soc. 1984;106:3319–3328. doi: 10.1021/ja00323a043. - DOI
LinkOut - more resources
Full Text Sources

