Twisting, Hydrogen Bonding, Exciplex Formation, and Charge Transfer. What Makes a Bright Fluorophore Not So Bright?
- PMID: 40464098
- PMCID: PMC12281576
- DOI: 10.1021/acs.joc.5c00492
Twisting, Hydrogen Bonding, Exciplex Formation, and Charge Transfer. What Makes a Bright Fluorophore Not So Bright?
Abstract
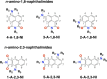
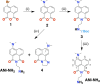
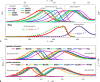
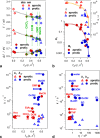
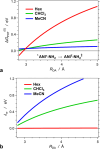
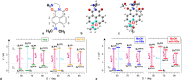
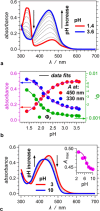
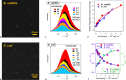
Excited-state charge transfer (CT) and the formation of twisted conformers govern the optical properties of a wide range of dyes. Aminonaphthalimide (ANI) derivatives, popular photosensitizers for blue light, are no exception. The focus herein is on an ANI derivative with an aliphatic amine, which ensures its solubility in a broad variety of solvents including water. Increasing solvent polarity quenches ANI fluorescence because of the expected formation of a dark twisted intramolecular charge-transfer (TICT) state. Contrary to the expectations, however, viscous polar alcohols, which inhibit conformational changes leading to the TICT state, only marginally recover ANI emission. Our analysis reveals that the "solubilizing" aliphatic amine forms an intramolecular exciplex, providing alternative nonradiative deactivation pathways without significant conformational changes and eliminating the viscosity dependence of ANI fluorescence. The findings also show a substantial dependence of ANI photophysics on hydrogen bonding with the solvating media, considerably enhancing the polarity effects. As a result, the fluorescence quantum yield of ANI (exceeding 0.4 in moderately polar solvents) drops below 0.001 when transferred to aqueous media. This feature allows one to showcase the utility of such dyes for imaging bacterial cells, complementing their growing popularity for CT, spintronics, materials, and biomedical applications.
Figures


Similar articles
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Stigma Management Strategies of Autistic Social Media Users.Autism Adulthood. 2025 May 28;7(3):273-282. doi: 10.1089/aut.2023.0095. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539215
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Liu T. L., Upadhyayula S., Milkie D. E., Singh V., Wang K., Swinburne I. A., Mosaliganti K. R., Collins Z. M., Hiscock T. W., Shea J.. et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science. 2018;360:eaaq1392. doi: 10.1126/science.aaq1392. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

