MyoMed205 Counteracts Titin Hyperphosphorylation and the Expression of Contraction-Regulating Proteins in a Rat Model of HFpEF
- PMID: 40464169
- PMCID: PMC12134774
- DOI: 10.1002/jcsm.13843
MyoMed205 Counteracts Titin Hyperphosphorylation and the Expression of Contraction-Regulating Proteins in a Rat Model of HFpEF
Abstract
Background: Heart failure with preserved ejection fraction (HFpEF) is associated with exercise intolerance, accompanied by alterations in the peripheral skeletal muscle (SKM). We have recently shown that titin, a giant sarcomere protein, is hyperphosphorylated in HFpEF. MuRF1 is a muscle-specific ubiquitin E3-ligase that interacts with titin. Blocking this interaction via small molecules (MyoMed205) can improve muscle function and mitochondrial activity in HFpEF. This study aimed to investigate the impact of MyoMed205 on titin phosphorylation and its association with changes in muscle structure and function.
Methods: Obese ZSF1 rats with established HFpEF received rat chow with (n = 15) or without (n = 15) MyoMed205 and were compared with lean littermates (n = 15), serving as controls. After 12 weeks, in vitro SKM force, atrophy and titin-as well as contractile protein expression-were evaluated (soleus and extensor digitorum longus [EDL]). Statistical analysis was performed via multiple unpaired t-test or one-way ANOVA.
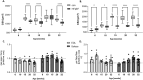
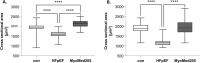
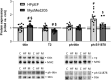
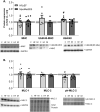
Results: In HFpEF, titin hyperphosphorylation by 13% in the EDL (p = 0.09) and 14% (p = 0.03) in the soleus muscle was evident. This hyperphosphorylation was driven in part by an increase in S11878 phosphorylation (EDL: +68%, p = 0.004; Sol: +23.8%, p = 0.03), which was linked to myofiber atrophy (r = -0.68, p = 0.006) and a decline in maximal specific muscle force (r = -0.54, p = 0.008). In the EDL, significant changes in protein expression related to atrophy (MuRF1 [+24.9%, p = 0.02], GDF8 [+20.6%, p = 0.09]) and calcium handling (slow troponin C [-46%, p = 0.02], fast troponin I [+35.8%, p = 0.02]) were found in HFpEF. All of the above-mentioned effects in HFpEF were almost completely abolished by MyoMed205 treatment, and significantly elevated titin expression was visible (+19.7%, pcon = 0.04, pHFpEF = 0.01).
Conclusions: Titin hyperphosphorylation may negatively impact skeletal muscle integrity and function in HFpEF. MyoMed205 reduced titin hyperphosphorylation and was associated with preserved skeletal muscle function and mass. Further studies are necessary to confirm the direct role of titin hyperphosphorylation on muscle function and to evaluate the therapeutic potential of MyoMed205 in HFpEF.
Keywords: HFpEF; MuRF1; MyoMed205; ZSF1; atrophy; contractile proteins; sarcopenia; skeletal muscle function; titin.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
V. A. and S. L. report a patent for MyoMed‐205, ID#704946 and further derivatives for its application to chronic muscle stress states (patent accession No. WO2021023643A1). B. V., A. S., A. A., M. E. P. J., P. B.; A. M. and T. S. B. declare no conflicts of interest. N. M. reports personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Bayer, Abbott, Abiomed and Boston Scientific, outside the submitted work. A. L. reports grants from Novartis, personal fees from Medtronic, Abbott, Edwards Lifesciences, Boston Scientific, Astra Zeneca, Novartis, Pfizer, Abiomed, Bayer, Boehringer and other from Picardia, Transverse Medical, Claret Medical, outside the submitted work. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Targeting MuRF1 by small molecules in a HFpEF rat model improves myocardial diastolic function and skeletal muscle contractility.J Cachexia Sarcopenia Muscle. 2022 Jun;13(3):1565-1581. doi: 10.1002/jcsm.12968. Epub 2022 Mar 17. J Cachexia Sarcopenia Muscle. 2022. PMID: 35301823 Free PMC article.
-
Modulation of Titin and Contraction-Regulating Proteins in a Rat Model of Heart Failure with Preserved Ejection Fraction: Limb vs. Diaphragmatic Muscle.Int J Mol Sci. 2024 Jun 16;25(12):6618. doi: 10.3390/ijms25126618. Int J Mol Sci. 2024. PMID: 38928324 Free PMC article.
-
Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction.Int J Mol Sci. 2025 Jun 27;26(13):6196. doi: 10.3390/ijms26136196. Int J Mol Sci. 2025. PMID: 40649974 Free PMC article.
-
Skeletal muscle alterations in HFrEF vs. HFpEF.Curr Heart Fail Rep. 2017 Dec;14(6):489-497. doi: 10.1007/s11897-017-0361-9. Curr Heart Fail Rep. 2017. PMID: 28940089 Review.
-
Skeletal muscle abnormalities in heart failure with preserved ejection fraction.Heart Fail Rev. 2023 Jan;28(1):157-168. doi: 10.1007/s10741-022-10219-9. Epub 2022 Mar 30. Heart Fail Rev. 2023. PMID: 35353269 Review.
References
-
- Borlaug B. A., Sharma K., Shah S. J., and Ho J. E., “Heart Failure With Preserved Ejection Fraction,” Journal of the American College of Cardiology 81 (2023): 1810–1834. - PubMed
-
- Redfield M. M. and Borlaug B. A., “Heart Failure With Preserved Ejection Fraction,” JAMA 329 (2023): 827. - PubMed
-
- Pieske B., Tschöpe C., de Boer R. A., et al., “How to Diagnose Heart Failure With Preserved Ejection Fraction: The HFA–PEFF Diagnostic Algorithm: A Consensus Recommendation From the Heart Failure Association (HFA) of the European Society of Cardiology (ESC),” European Heart Journal 40 (2019): 3297–3317. - PubMed
-
- Del Buono M. G., Arena R., Borlaug B. A., et al., “Exercise Intolerance in Patients With Heart Failure,” Journal of the American College of Cardiology 73 (2019): 2209–2225. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

